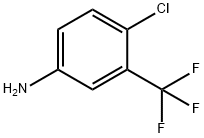
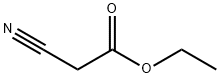
N-[4-chloro-3-(trifluoromethyl)phenyl]-2-cyanoacetamide synthesis
- Product Name:N-[4-chloro-3-(trifluoromethyl)phenyl]-2-cyanoacetamide
- CAS Number:24522-41-6
- Molecular formula:C10H6ClF3N2O
- Molecular Weight:262.62
Yield:24522-41-6 26%
Reaction Conditions:
Stage #1: cyanoacetic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 1 h;
Stage #2: 4-chloro-3-trifluoromethyl-anilinewith N-ethyl-N,N-diisopropylamine in dichloromethane at 20;
Steps:
1.A Step A. N- [4- Chloro-3-(trifluoromethyl)phenyl] -2-cyano-acetamide
Cyanoacetic acid (283 mg; 3.3 mmol) was dissolved in DCM (6.00 ml) and two drops of DMF followed by oxalyl chloride (430 jil; 5.00 mmol) were successively added (gas release). The colorless mixture was stuffed for lh at rt then concentrated in vacuo to give a black oil. 4-Chloro- 3-(trifluoromethyl)aniline (0.37 ml; 3.00 mmol) and DIEA (660 jil; 4.00 mmol) were added at rt to the black residue previously taken up in 6 mL of DCM. The black mixture was stuffed at rtovernight. The reaction was diluted with 20 mL of DCM, quenched by the addition of Aq NaHCO3 and extracted with DCM. The combined organic phase was dried with MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (eluting with 25-50% EtOAc in cyclohexane) to give N-[4-chloro-3-(trifluoromethyl)phenyl]-2 -cyano-acetamide (0.229 g, 26%) as a pale yellow solid. ‘H NMR (DMSO-d6, 300 MHz): ? = 10.74 (s, 1H), 8.11 (d, J=2.5Hz, 1H), 7.79 (dd, J=8.6, 2.5 Hz, 1H), 7.70 (d, J=8.6 Hz, 1H), 3.96 ppm (s, 2H). LC/MS (Table 1, Method A) R = 1.93 mm; MS m/z: 261 [M-H].
References:
WO2018/122317,2018,A1 Location in patent:Page/Page column 129; 130