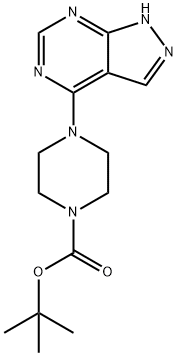
4-(1H-Pyrazolo[3,4-d]pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-(1H-Pyrazolo[3,4-d]pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester
- CAS Number:245450-02-6
- Molecular formula:C14H20N6O2
- Molecular Weight:304.35
![4-Chloro-1H-pyrazolo[3,4-d]pyrimidine](/CAS/GIF/5399-92-8.gif)
5399-92-8
232 suppliers
$13.43/250mgs:

57260-71-6
738 suppliers
$5.00/5g
![4-(1H-Pyrazolo[3,4-d]pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester](/CAS/GIF/245450-02-6.gif)
245450-02-6
7 suppliers
inquiry
Yield:245450-02-6 89%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 90; for 4 h;
Steps:
1 Preparation of Intermediate 2
The starting material B-Cl(1a-n) (12.94 mmol) was weighed and dissolved in DMF (10 mL).DIEA (51.76mmol),1-Boc piperazine (15.53 mmol) was added to the reaction flask and reacted at 90 ° C for 4 h.The reaction was completely detected by TLC, and the reaction solution was poured into 10 times of ice water.A large amount of white solid was precipitated, filtered, and the cake was washed with water (20 ml × 3) and dried.Intermediate 2 was obtained. In the above preparation, la is 4-chloro-1H-pyrazolo[3,4-d]pyrimidine, which gives 2a as a white solid, yield 89%
References:
CN108822110,2018,A Location in patent:Paragraph 0112-0116
![2H-Pyrazolo[3,4-d]pyrimidin-4-amine (9CI)](/CAS/GIF/64834-00-0.gif)
64834-00-0
7 suppliers
inquiry

57260-71-6
738 suppliers
$5.00/5g
110-46-3
289 suppliers
$10.00/10g
![4-(1H-Pyrazolo[3,4-d]pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester](/CAS/GIF/245450-02-6.gif)
245450-02-6
7 suppliers
inquiry
315-30-0
763 suppliers
$5.00/1g
![4-(1H-Pyrazolo[3,4-d]pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester](/CAS/GIF/245450-02-6.gif)
245450-02-6
7 suppliers
inquiry
![4-Aminopyrazolo[3,4-d]pyrimidine](/CAS/GIF/2380-63-4.gif)