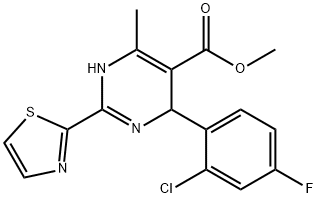
methyl 4-(2-chloro-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate synthesis
- Product Name:methyl 4-(2-chloro-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate
- CAS Number:247037-77-0
- Molecular formula:C16H13ClFN3O2S
- Molecular Weight:365.81
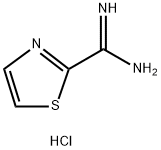
247037-82-7
147 suppliers
$6.00/250mg
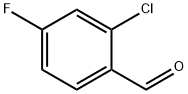
84194-36-5
337 suppliers
$9.00/1g
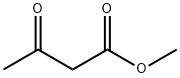
105-45-3
650 suppliers
$5.00/5g

247037-77-0
7 suppliers
inquiry
Yield:247037-77-0 79.2%
Reaction Conditions:
Stage #1: 2-chloro-4-fluorobenzaldehyde;acetoacetic acid methyl esterwith piperidine;acetic acid in isopropyl alcohol at 43 - 47; for 5 h;Large scale;
Stage #2: 1,3-thiazole-2-carboximidamide hydrochloridewith triethylamine in isopropyl alcohol at 80 - 85; for 7 h;Large scale;
Steps:
1 Example 1Preparation of 4-(2-chloro-4-fluoro-phenyl)-6-methyl-2-thiazol-2-yl- 1,4-dihydropyrimidine- 5-carboxylic acid methyl ester:
To a 1000 L glass-lined reactor was charged 2-chloro-4-fluoro-benzaldehyde (30.8 kg,194 mol) and isopropanol (188.0 kg). To the solution was then added methyl acetoacetate (22.7 kg, 195 mol) followed by piperidine (1.74 kg, 20.4 mol) and acetic acid (1.32 kg, 22.0 mol). The mixture was then heated to 43°C - 47 °C and stirred under this temperature for 5 hours. To the reaction mixture was then added thiazole-2-carboxamidine hydrochloride salt (19.8 kg, 121.Omol)and triethyl amine (20.0 kg, 198.0 mol). The reaction mixture was heated to 80 °C - 85 °C and stirred for 7 hours.After reaction completion, the reaction mixture was cooled to 20 °C - 25 °C. To the mixture was then added water (52.0 kg). The resulting suspension was stirred at 20 °C - 25 °C for 2 hours. The suspension was centrifuged and the collected solid was washed with isopropanollwater (42kg; lOv/3v). The wet solid was dried in vacuum oven to afford 35.05 kg of 4-(2-chloro-4-fluoro- phenyl)-6-methyl-2-thiazol-2-yl- 1 ,4-dthydropyrimidine-5-carboxylic acid methyl ester (Example1). The purity was 95.8%, the yield was 79.2 %, and MS mle = 366.2 [M+H] .
References:
WO2016/102438,2016,A1 Location in patent:Page/Page column 11

298709-35-0
5 suppliers
inquiry

247037-82-7
147 suppliers
$6.00/250mg

247037-77-0
7 suppliers
inquiry

247037-82-7
147 suppliers
$6.00/250mg
84194-30-9
180 suppliers
$14.00/1g

105-45-3
650 suppliers
$5.00/5g

247037-77-0
7 suppliers
inquiry

247037-82-7
147 suppliers
$6.00/250mg

30414-53-0
313 suppliers
$5.00/1g

84194-36-5
337 suppliers
$9.00/1g

247037-77-0
7 suppliers
inquiry