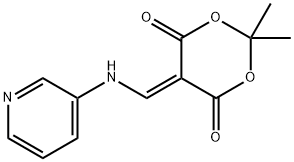
2,2-Dimethyl-5-(pyridin-3-ylaminomethylene)-[1,3]dioxane-4,6-dione synthesis
- Product Name:2,2-Dimethyl-5-(pyridin-3-ylaminomethylene)-[1,3]dioxane-4,6-dione
- CAS Number:25063-68-7
- Molecular formula:C12H12N2O4
- Molecular Weight:248.23
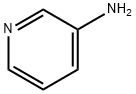
462-08-8
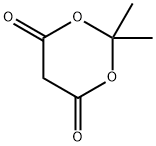
2033-24-1
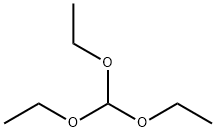
122-51-0
![2,2-Dimethyl-5-(pyridin-3-ylaminomethylene)-[1,3]dioxane-4,6-dione](/CAS/20180703/GIF/25063-68-7.gif)
25063-68-7
To a preheated mixture of 3-aminopyridine (0.37 g, 4.0 mmol) and 2,2-dimethyl-1,3-dioxane-4,6-dione (Meldrum's acid, 0.69 g, 4.8 mmol) was added triethyl orthoformate (4.0 mL, 24.0 mmol). The reaction mixture was stirred at 100 °C for 2 h. The formation of a yellow precipitate was observed during the reaction and the color of the solution gradually changed from yellow to burgundy. Upon completion of the reaction, the mixture was cooled to room temperature and the excess triethyl orthoformate was subsequently removed by vacuum distillation. The resulting solid was purified by silica gel column chromatography with the eluent being a gradient of 70% to 100% ethyl acetate in hexane solution.

462-08-8
473 suppliers
$14.00/25g

2033-24-1
656 suppliers
$6.00/25g

122-51-0
510 suppliers
$10.00/5ml
![2,2-Dimethyl-5-(pyridin-3-ylaminomethylene)-[1,3]dioxane-4,6-dione](/CAS/20180703/GIF/25063-68-7.gif)
25063-68-7
24 suppliers
$45.00/100mg
Yield:25063-68-7 90%
Reaction Conditions:
Stage #1: cycl-isopropylidene malonate;orthoformic acid triethyl ester at 90; for 1.5 h;Large scale;
Stage #2: pyridin-3-ylamine in ethanol at 60 - 70; for 0.666667 h;Large scale;
Steps:
2,2-dimethyl-5-((pyridin-3-ylamino)methylene)-1,3-dioxane-4,6-dione (112)
(0527) A mixture of triethyl orthoformate (1.665 mL, 10.0 mmol) and 2,2-dimethyl-1,3-dioxane-4,6-dione (865 mg, 6.0 mmol) was heated at 90°C for 1.5 h and then cooled to 70°C. 3-amino- pyridine 15 (471 mg, 5.0 mol) was slowly added over 10 min with an EtOH (20 mL) rinse while maintaining the reaction temperature between 60 and 70°C. The reaction was then heated for an additional 30 min and allowed to cool to RT. The precipitate was filtered, washed with EtOH (20 mL), and dried to yield compound as a light-yellow solid (1118mg, 90%). 1H NMR (400 MHz, chloroform-i δ 1 1.25 (d, J= 14.2 Hz, 1H), 8.66 - 8.59 (m, 2H), 8.55 (dd, J= 4.8, 1.4 Hz, 1H), 7.61 (ddd, J= 8.3, 2.8, 1.4 Hz, 1H), 7.41 (dd, J = 8.3, 4.7 Hz, 1H), 1.77 (s, 6H).
References:
WO2019/89648,2019,A1 Location in patent:Page/Page column 67