
ETHYL 2-[1-(4-NITROPHENYL)-4-PIPERIDINYLIDENE]ACETATE synthesis
- Product Name:ETHYL 2-[1-(4-NITROPHENYL)-4-PIPERIDINYLIDENE]ACETATE
- CAS Number:251310-52-8
- Molecular formula:C15H18N2O4
- Molecular Weight:290.31

23499-01-6
36 suppliers
$15.00/250mg
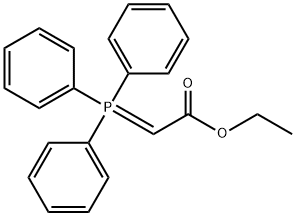
1099-45-2
372 suppliers
$6.00/5g
![ETHYL 2-[1-(4-NITROPHENYL)-4-PIPERIDINYLIDENE]ACETATE](/StructureFile/ChemBookStructure2/GIF/CB3329966.gif)
251310-52-8
8 suppliers
inquiry
Yield:251310-52-8 82%
Reaction Conditions:
in benzene; for 24 h;Product distribution / selectivity;Heating / reflux;
Steps:
Scheme 4a
References:
WO2008/130637,2008,A1 Location in patent:Page/Page column 32-33
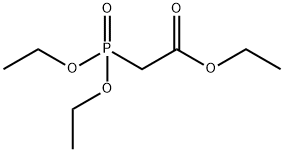
867-13-0
474 suppliers
$10.00/1g

23499-01-6
36 suppliers
$15.00/250mg
![ETHYL 2-[1-(4-NITROPHENYL)-4-PIPERIDINYLIDENE]ACETATE](/StructureFile/ChemBookStructure2/GIF/CB3329966.gif)
251310-52-8
8 suppliers
inquiry
![1,4-Dioxa-8-azaspiro[4.5]decane, 8-(4-nitrophenyl)-](/CAS/20180601/GIF/79421-42-4.gif)
79421-42-4
3 suppliers
inquiry
![ETHYL 2-[1-(4-NITROPHENYL)-4-PIPERIDINYLIDENE]ACETATE](/StructureFile/ChemBookStructure2/GIF/CB3329966.gif)
251310-52-8
8 suppliers
inquiry
![1,4-Dioxa-8-azaspiro[4.5]decane](/CAS/GIF/177-11-7.gif)
177-11-7
289 suppliers
$6.00/1g
![ETHYL 2-[1-(4-NITROPHENYL)-4-PIPERIDINYLIDENE]ACETATE](/StructureFile/ChemBookStructure2/GIF/CB3329966.gif)
251310-52-8
8 suppliers
inquiry
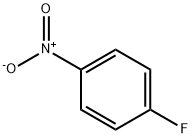
350-46-9
515 suppliers
$10.60/1gm:
![ETHYL 2-[1-(4-NITROPHENYL)-4-PIPERIDINYLIDENE]ACETATE](/StructureFile/ChemBookStructure2/GIF/CB3329966.gif)
251310-52-8
8 suppliers
inquiry