
METHYL 4-CHLORO-7H-CYCLOPENTA[C]PYRIDINE-6-CARBOXYLATE synthesis
- Product Name:METHYL 4-CHLORO-7H-CYCLOPENTA[C]PYRIDINE-6-CARBOXYLATE
- CAS Number:251996-85-7
- Molecular formula:C9H6ClNO2S
- Molecular Weight:227.67
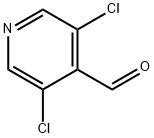
136590-83-5
149 suppliers
$6.00/250mg

2365-48-2
342 suppliers
$14.00/25g
![METHYL 4-CHLORO-7H-CYCLOPENTA[C]PYRIDINE-6-CARBOXYLATE](/CAS/GIF/251996-85-7.gif)
251996-85-7
25 suppliers
$65.00/50mg
Yield:251996-85-7 88%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20; for 3 h;
Steps:
8
Methyl 4-chlorothieno[2,3-c]pyridine-2-carboxylate. 3,5-Dichloroisonicotinaldehyde (20.39 g, 115.8 mmol) obtained from Aldrich was dissolved in 250 mL DMF. To this solution was added Cs2CO3 (12.9 g, 121.59 mmol) and then
References:
WO2009/11871,2009,A2 Location in patent:Page/Page column 130-131
2457-47-8
298 suppliers
$6.00/5g
![METHYL 4-CHLORO-7H-CYCLOPENTA[C]PYRIDINE-6-CARBOXYLATE](/CAS/GIF/251996-85-7.gif)
251996-85-7
25 suppliers
$65.00/50mg