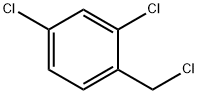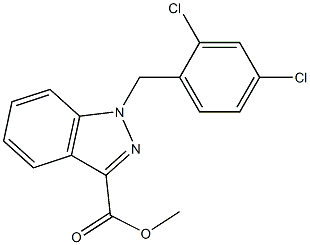
Methyl 1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylate synthesis
- Product Name:Methyl 1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylate
- CAS Number:252025-50-6
- Molecular formula:C16H12Cl2N2O2
- Molecular Weight:335.1847
Yield:252025-50-6 89%
Reaction Conditions:
with potassium carbonate in acetone at 70;
Steps:
1
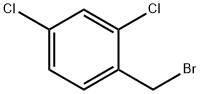
Next, a mixture of methyl indazole-3-carboxylate (2.05g, 11.4mmol), 2,4- dichlorobenzyl chloride (3.35mL, 12.54 mml), and K2CO3 (7.Og, 50mmol) in acetone (22mL) was refluxed overnight at a temperature of 70 0C. The reaction mixture was cooled to room temperature, filtered, and the residue was washed with acetone. The combined filtrate was concentrated under vacuum (rotovapor). The solid thus obtained was dissolved in CH2Cl2 and filtered to remove any undissolved solid. The solution was then concentrated, diluted with hexane and left in the refrigerator overnight. The precipitated solid was then filtered, washed with a mixture of hexane/ethyl acetate (9: 1) to yield the pure product as a white solid. Yield = 3.5g (89%); m.p. = 144°-146 0C; IH NMR (400MHz, CDCl3) D 8.31 (d, J = 8.8 Hz, IH), 7.39-7.47 (m, 4H), 7.12 (d, J = 8.8 Hz, IH), 6.71 (d, J = 8.8 Hz, IH), 5.81 (s, 2H), 4.11 (s, 3H).
References:
WO2011/5759,2011,A2 Location in patent:Page/Page column 70
20443-99-6
106 suppliers
$25.00/1g

43120-28-1
166 suppliers
$10.00/1g

252025-50-6
22 suppliers
inquiry

67-56-1
758 suppliers
$9.00/25ml

50264-69-2
226 suppliers
$10.00/5mg

252025-50-6
22 suppliers
inquiry
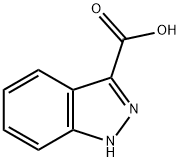
4498-67-3
477 suppliers
$6.00/5g

252025-50-6
22 suppliers
inquiry