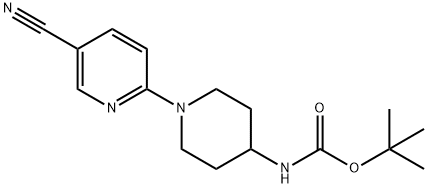
tert-Butyl n-[1-(5-cyanopyridin-2-yl)piperidin-4-yl]carbamate synthesis
- Product Name:tert-Butyl n-[1-(5-cyanopyridin-2-yl)piperidin-4-yl]carbamate
- CAS Number:252577-86-9
- Molecular formula:C16H22N4O2
- Molecular Weight:302.37
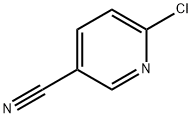
33252-28-7
433 suppliers
$6.00/1g
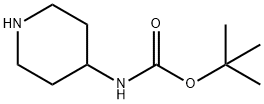
73874-95-0
454 suppliers
$10.00/250mg
![tert-Butyl n-[1-(5-cyanopyridin-2-yl)piperidin-4-yl]carbamate](/CAS/20210111/GIF/252577-86-9.gif)
252577-86-9
4 suppliers
inquiry
Yield:252577-86-9 99%
Reaction Conditions:
with sodium carbonate in N,N-dimethyl-formamide at 90; for 4 h;
Steps:
3.1 tert-butyl (1-(5-cyanopyridin-2-yl)piperidin-4-yl)carbamate
A solution of tert- vXy piperidin-4-ylcarbamate (3.00 g, 15.00 mmol), 6- chloronicotinonitrile (2.08 g, 15.00 mmol) and Na2C03(3.20 g, 30.19 mmol) in DMF (40 mL) was heated to 90 °C and stirred for 4 h. The reaction mixture was cooled to room temperature, diluted with water (120 mL), and extracted with EtOAc (100 mL x 3). The combined organic phases were washed with brine (300 mL), dried over anhydrous Na2S04, filtered and concentrated in vacuo. The residue was washed with PE/EtOAc (10/1 (v/v), 80 mL) to give the title compound as a white solid (4.50 g, 99 %).MS ( ESI, pos. ion) m/z: 247.0 [M-C4H8+ H]+; H NMR (400 MHz, CDCb): δ (ppm) 8.40 (d, J = 2.36 Hz, 1H), 7.62-7.59 (dd, J = 2.36 Hz, 9.08 Hz, 1H), 6.63 (d, J = 9.08 Hz, 1H), 4.45 (m, 1H), 4.36-4.33 (m, 2H), 3.75 (m, 1H), 3.14-3.07 (m, 2H), 2.08-2.06 (m, 2H), 1.45 (s, 9H), 1.43-1.37 (m, 2H).
References:
WO2015/73267,2015,A1 Location in patent:Paragraph 324