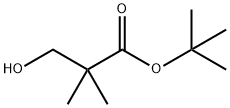
Propanoic acid, 3-hydroxy-2,2-diMethyl-, 1,1-diMethylethyl ester synthesis
- Product Name:Propanoic acid, 3-hydroxy-2,2-diMethyl-, 1,1-diMethylethyl ester
- CAS Number:25307-76-0
- Molecular formula:C9H18O3
- Molecular Weight:174.24
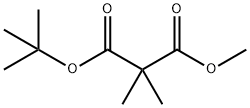
85293-46-5
15 suppliers
inquiry

25307-76-0
6 suppliers
inquiry
Yield:25307-76-0 47%
Reaction Conditions:
with diisobutylaluminium hydride in tetrahydrofuran;toluene at -78 - 20;Inert atmosphere;
Steps:
Intermedaiate 2b: tert-Butyl 3-hydroxy-2,2-dimethylpropanoate
To a solution of 1-tert-butyl 3-methyl 2,2-dimethylmalonate 2a (5.00 g, 24.7 mmol) in tetrahydrofuran (30 mL) was added 1.5 M diisobutylaluminum hydride in toluene (41.3 mL, 61.9 mmol) dropwise at - 78 °C under nitrogen atmosphere. After stirred at thistemperature under nitrogen atmosphere for 2 hours and then at room temperature overnight, the mixture was quenched with water (50 mL) and extracted with ethyl acetate (100 mL) for three times. The combined organic layers were washed with water (200 mL) for three times and brine (100 mL), dried over Na2SO4() and filtered. The filtrate was concentrated under reduced pressure to give the title compound (2.00 g, 47 %yield) as white oil. ‘H NIVIR (300 1VIHz, CDC13) 3.52 (d, J 5.4 Hz, 2H), 2.58 (br s,1H), 1.47 (s, 9H), 1.16 (s, 6H).
References:
WO2019/1420,2019,A1 Location in patent:Page/Page column 89