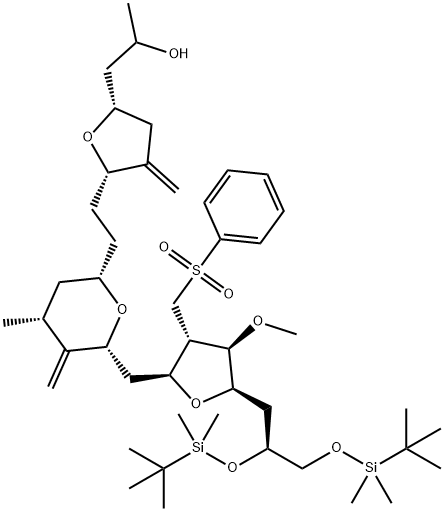
(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl] synthesis
- Product Name:(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]
- CAS Number:253128-10-8
- Molecular formula:C45H78O9SSi2
- Molecular Weight:851.33
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]](/CAS/20200611/GIF/253128-10-8.gif)
253128-10-8
27 suppliers
inquiry
Yield: 87%
Reaction Conditions:
Stage #1:C52H90O12SSi2 with pyridine;Methanesulfonic anhydride in dichloromethane at 0 - 20; for 1 h;
Stage #2: with methanol;sodium methylate in tetrahydrofuran at 0 - 20; for 1 h;Williamson Synthesis;
Steps:
AE
Compound AEZinc powder (1.54 g, 23.6 mmol, 50 eq) was suspended in water (1.1 ml) and cooled to 0° C. AcOH (0.40 ml, 7.1 mmol, 15 eq) was added at 0° C. A solution of Compound AF (530 mg, 0.473 mmol, 1 eq) in THF (2.7 ml) was added at 0° C., and the mixture was allowed to warm to 20° C. After 3 h, the reaction mixture was filtered for removal of zinc powder. The reactor was rinsed with a mixture of THF (1.1 ml) and water (1.1 ml). The filtrate was diluted with MTBE (10.6 ml), sequentially washed with: 1) 20 wt % Rochelle salt (aqueous solution, 2.7 g, 4.0 eq), 2) saturated NaHCO3 (6.0 g), and 3) 20 wt % NaCl (aqueous solution, 2.6 g), and concentrated to give crude product as colorless oil. The crude was purified by Biotage (Uppsala, Sweden) 25M (heptane-MTBE 1:1 v/v) to give Compound AE (393 mg, 0.394 mmol, 83% yield) as colorless oil. 1H NMR (400 MHz, CDCl3): δ 7.93 (2H, m), 7.66 (1H, m), 7.58 (2H, m), 5.23 (1H, t, J=6.4 Hz), 5.05 (1H, s), 4.95 (1H, d, J=1.6 Hz), 4.88 (1H, s), 4.83 (1H, d, J=1.6 Hz), 4.33 (1H, br), 4.02 (3H, m), 3.83 (2H, m), 3.76 (1H, m), 3.60 (1H, m), 3.54 (1H, dd, J=5.6 Hz, 10.4 Hz), 3.47 (2H, m), 3.37 (3H, s), 3.15 (1H, dd, J=4.0 Hz, 14.0 Hz), 2.95 (1H, dd, J=10.0 Hz, 14.0 Hz), 2.83 (1H, d, J=5.2 Hz), 2.65 (2H, m), 2.40 (1H, m), 2.23 (1H, m), 2.03 (3H, s), 1.96-2.03 (2H, m), 1.81 (1H, m), 1.67-1.80 (3H, m), 1.40-1.67 (7H, m), 1.17 (9H, s), 1.01 (3H, d, J=6.8 Hz), 0.86 (18H, s), 0.06 (6H, s), 0.03 (6H, s); 13C NMR (100 MHz, CDCl3): δ 178.80, 170.77, 153.18, 151.49, 139.77, 134.16, 129.67 (2C), 128.16 (2C), 109.77, 105.27, 85.84, 80.92, 80.15, 78.57, 76.97, 74.59, 71.51, 68.80, 68.05, 64.43, 58.01, 57.56, 45.21, 43.49, 39.78, 38.94, 34.58, 33.55, 32.28, 31.77, 31.74, 27.42 (3C), 26.21 (3C), 26.17 (3C), 25.49, 22.78, 21.51, 18.60, 18.39, -3.87, -4.51, -5.11 (2C).ER-804028Compound AE (280 mg, 0.281 mmol, 1 eq) was dissolved in CH2Cl2 and cooled to 0° C. Pyridine (0.045 ml, 0.56 mmol, 2.0 eq) was added followed by Ms2O (58.8 mg, 0.338 mmol, 1.20 eq). The reaction was allowed to warm to room temperature, and stirring was continued for an additional 1 h. The reaction mixture was cooled to 0° C., diluted with MTBE (5.6 ml), washed with saturated NaHCO3 (0.84 g), and concentrated to give crude product as colorless film. The crude was azeotropically dried with heptane (3 ml×2) and re-dissolved in THF (7.0 ml). The mixture was cooled to 0° C. and treated with 25 wt % NaOMe (0.13 ml). After 10 min, the reaction was allowed to warm to room temperature, and stirring was continued for an additional 30 min. The mixture was treated with additional 25 wt % NaOMe (0.045 ml), and stirring was continued for an additional 20 min. The reaction mixture was diluted with heptane (7.0 ml) and washed with water (1.4 ml). The organic layer was separated, sequentially washed with: 1) 20 wt % NH4Cl (0.84 g) and 2) 20 wt % NaCl (3 g), and concentrated to give crude product as brownish oil. The crude was purified by Biotage (Uppsala, Sweden) 12M (heptane-MTBE 2:3 v/v) to give ER-804028 (209 mg, 0.245 mmol, 87%) as pale yellow oil. 1H NMR (400 MHz, CDCl3): δ 7.89 (2H, m), 7.64 (1H, m), 7.56 (2H, m), 4.85 (1H, d, J=1.6 Hz), 4.80 (1H, s), 4.72 (1H, s), 4.61 (1H, d, J=1.6 Hz), 4.23 (1H, br), 3.91 (1H, m), 3.79 (1H, m), 3.76 (2H, m), 3.63 (1H, m), 3.50-3.60 (4H, m), 3.43 (1H, dd, J=5.6 Hz, 10.0 Hz), 3.38 (3H, s), 3.32 (1H, m), 2.98 (2H, m), 2.61 (1H, br), 2.56 (1H, m), 2.50 (1H, m), 2.08-2.22 (3H, m), 1.96 (1H, m), 1.84 (1H, m), 1.78 (1H, m), 1.70 (1H, m), 1.42-1.63 (6H, m), 1.28-1.42 (2H, m), 1.01 (3H, d, J=6.8 Hz), 0.84 (18H, s), 0.05 (3H, s), 0.04 (3H, s), 0.00 (3H, s), -0.01 (3H, s); and 13C NMR (100 MHz, CDCl3): δ 150.34, 150.75, 139.91, 134.18, 129.73 (2C), 128.14 (2C), 105.10, 85.97, 80.92, 79.72, 78.50, 77.45, 77.09, 75.53, 71.59, 68.04, 62.88, 58.27, 57.73, 43.51, 42.82, 39.16, 37.68, 35.69, 33.31, 32.41, 31.89, 31.48, 29.79, 26.21 (3C), 26.17 (3C), 18.58, 18.38, 18.13, -3.85, -4.71, -5.12 (2C).
References:
Eisai R&D Management Co., Ltd. US2011/184190, 2011, A1 Location in patent:Page/Page column 18-20
![Propanoic acid, 2,2-dimethyl-, 3-[(2S,5S)-5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl)methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-2-furanyl]propyl ester](/CAS/20200611/GIF/253128-09-5.gif)
253128-09-5
0 suppliers
inquiry
![(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]](/CAS/20200611/GIF/253128-10-8.gif)
253128-10-8
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]](/CAS/20200611/GIF/253128-10-8.gif)
253128-10-8
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]](/CAS/20200611/GIF/253128-10-8.gif)
253128-10-8
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]](/CAS/20200611/GIF/253128-10-8.gif)
253128-10-8
27 suppliers
inquiry