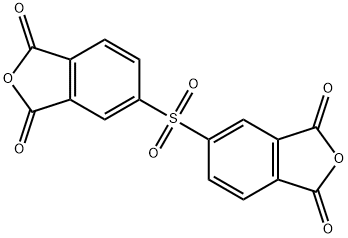
3,3,4,4-diphenylsulfonetetracarboxylicdianhydride synthesis
- Product Name:3,3,4,4-diphenylsulfonetetracarboxylicdianhydride
- CAS Number:2540-99-0
- Molecular formula:C16H6O8S
- Molecular Weight:358.28
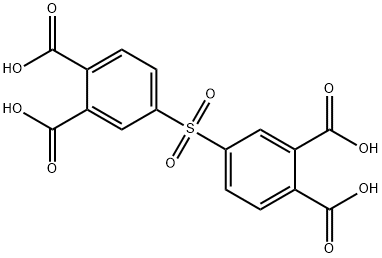
Then, the resulting 4,4'-Thiodiphthalic anhydride 20.0g (0.061 mol), 12-tungstophosphoric acid (H3PW12O40 · 30H2O) 1.0g and acetic acid 250g were charged to four-necked flask made of glass equipped with reflux condenser, thermometer, dropping funnel and stirrer, dissolved by heating to 110°C. Cooled to 80°C, under stirring, the reaction temperature was maintained at 80°C and 9% peracetic acid· acetic acid solution 77.5g (0.091 mol) was added dropwise and takes 4 hours, further reacted for 2 hours. The reaction mixture was cooled to 15°C, the obtained crystals were then filtered, under reduced pressure, and dried for 3 hours at 100°C. 4,4'-Sulfonyldiphthalic anhydride 19.9g (isolation yield 91%) was obtained. This thing was analyzed by HPLC as hydrolysis, as a result purity was 98.9%.

10595-31-0
5 suppliers
inquiry

2540-99-0
131 suppliers
$13.00/1g
Yield:2540-99-0 92%
Reaction Conditions:
with bathophenanthroline in N,N-dimethyl acetamide;toluene; for 12 h;Reflux;
Steps:
31 Example 25:
General procedure: In a 25ml three-necked flask, add 4,7-diphenyl-1,10-phenanthroline (17mg, 2mol%), 4-methylphthalic acid (450mg, 2.5mmol), and finally add solvent toluene (9mL) ) and N,N-dimethylacetamide (1 ml) to help dissolve, the mixture was heated under azeotropic reflux conditions for 12 hours, and the reflux liquid was passed through molecular sieves to remove water. After the reaction was completed, the mixture was cooled and filtered to remove the solvent to obtain 4-methylphthalic anhydride in a yield of 97%.
References:
CN114213372,2022,A Location in patent:Paragraph 0045-0050

25884-43-9
10 suppliers
inquiry

2540-99-0
131 suppliers
$13.00/1g