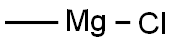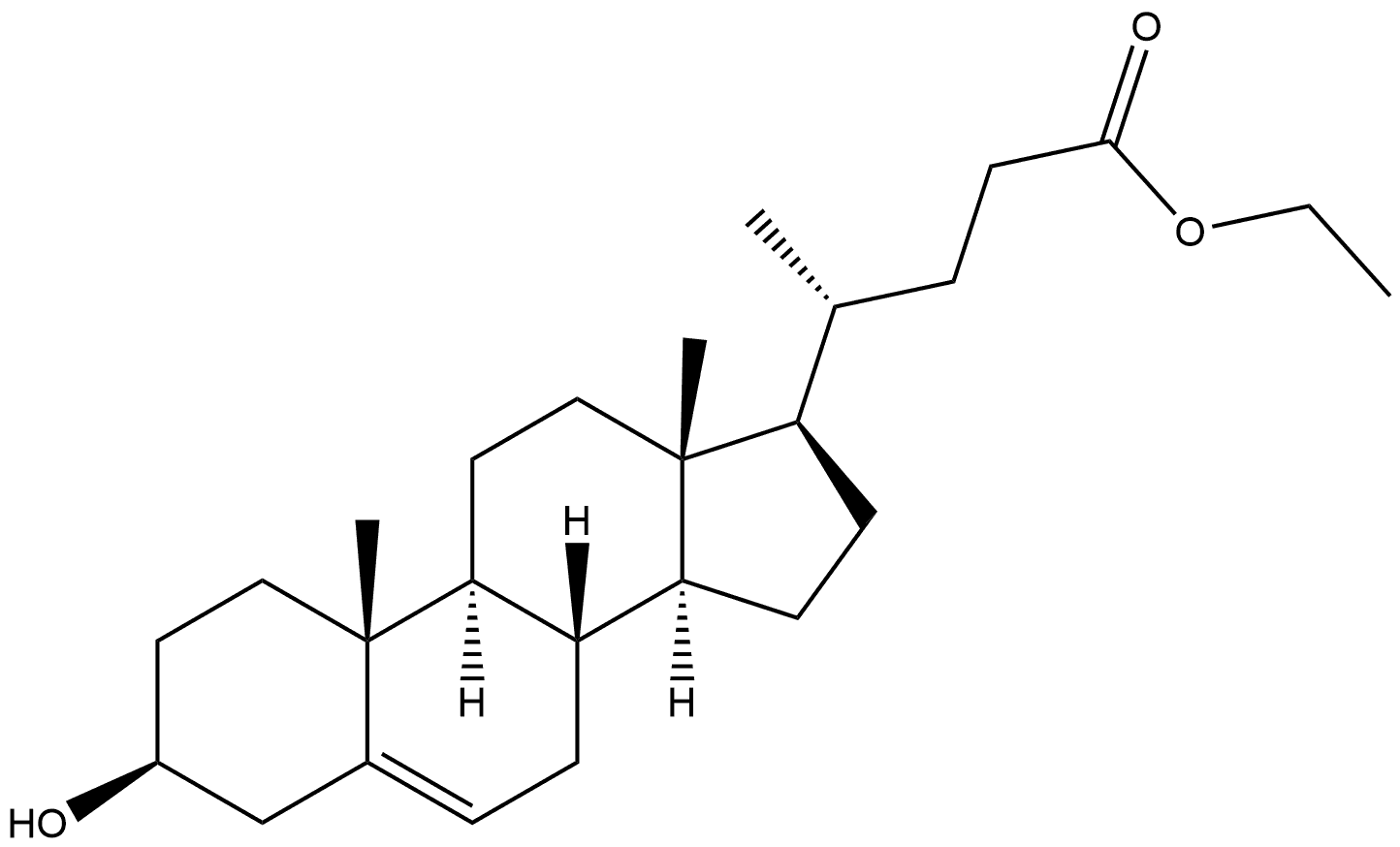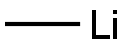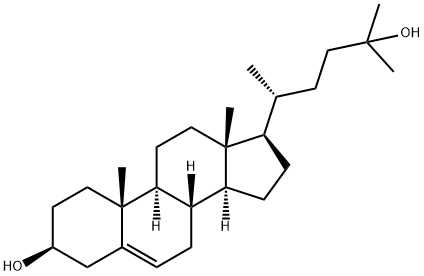
26,27-Dinorergost-5-ene-3β,24-diol synthesis
- Product Name:26,27-Dinorergost-5-ene-3β,24-diol
- CAS Number:35882-85-0
- Molecular formula:C26H44O2
- Molecular Weight:388.63
Yield:35882-85-0 97%
Reaction Conditions:
in tetrahydrofuran at 0 - 20; for 2.25 h;Inert atmosphere;
Steps:
15 [00373] Preparation of compound BB-2:
[00373] Preparation of compound BB-2: Under nitrogen a solution of BB-1 (1.75 g, 4.06 mmol) in THF (35 mL), prepared as described in Steroids (2006) 71: 18, was cooled to 0°C. Methylmagnesium chloride (22% (w/w) in THF, 19.5 mL, 58.1 mmol) was added dropwise. Stirring at 0°C was continued for 15 minutes and reaction mixture was allowed to warm to room temperature and stirring was continued for two hours. Saturated aqueous NH4CI (5 mL) was added slowly. Precipitate formed and was dissolved by addition of water (10 mL). EtOAc (50 mL) and brine (20 mL) were added. Layers were separated. Aqueous layer was extracted with EtOAc (2 x 50 mL). Combined organic layers were dried with Na2S04 and solvents were removed in vacuo. Residue was coevaporated with dichloromethane (50 mL). BB-2 (1.54 g, 3.95 mmol, 97%) was obtained as an off-white solid. 1HNMR (400 MHz, CDC13): 5(ppm): 5.32 - 5.43 (1H, m), 3.46 - 3.61 (1H, m), 1.20 (3H, s), 1.19 (3H, s), 1.01 (3H, s), 0.93 (3H, d, J = 6.6 Hz), 0.68 (3H, s).
References:
WO2013/36835,2013,A1 Location in patent:Paragraph 00373
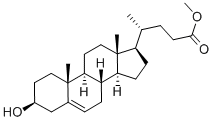
20231-57-6
27 suppliers
$225.00/100MG

75-16-1
268 suppliers
$12.00/10ml

35882-85-0
2 suppliers
inquiry

53906-49-3
7 suppliers
inquiry

35882-85-0
2 suppliers
inquiry
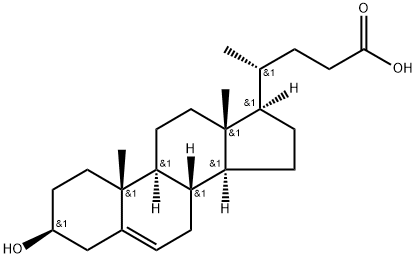
5255-17-4
83 suppliers
$60.00/10mg

35882-85-0
2 suppliers
inquiry