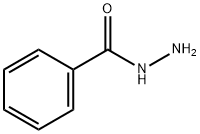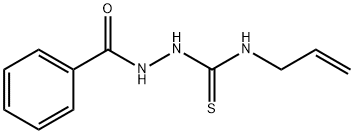
Benzoic acid, 2-[(2-propen-1-ylaMino)thioxoMethyl]hydrazide synthesis
- Product Name:Benzoic acid, 2-[(2-propen-1-ylaMino)thioxoMethyl]hydrazide
- CAS Number:26029-04-9
- Molecular formula:C11H13N3OS
- Molecular Weight:235.31
Yield:26029-04-9 85%
Reaction Conditions:
in ethanol; for 4 h;Reflux;
Steps:
2.1. Synthesis of the organic ligand
The ligand L was obtained in several steps as shown in Scheme 1. Benzhydrazide was prepared in two steps from benzoic acid by atypical procedure. Then a solution of allyl isothiocyanate (1.98 g, 20 mmol) in 10 mL of ethanol was added dropwise to the hot solution of benzhydrazide (2.72 g, 20 mmol) in 25 mL of ethanol. The prepared mixture was heated under reflux for 4 h and then ice cooled.The obtained white precipitate was filtered off and then recrystallized from ethanol to give N-allyl-2-benzoylhydrazinecarbothioamide as white needles. Yield: 4.0 g (85%), m.p. 185 °C. This synthon was subjected to alkaline cyclization similarly to the described procedure [21]. A solution of 27.3 g.(0.116 mol) of N-allyl-2-benzoylhydrazinecarbothioamide and4.8 g. (0.12 mol) of sodium hydroxide in 35 ml of water in a roundbottomedflask is heated on a steam bath for 1 h. The solution iscooled for 30 min in an ice bath and then is treated with 11 ml ofconcentrated hydrochloric acid. The reaction mixture is cooled inan ice bath for 2 h, and the product that precipitates is collected bysuction filtration, washed with ice-water and air-dried overnight.3-Thiol-4-allyl-5-phenyl-4H-1,2,4-triazole weighs 20.1 g. (50%) andmelts at 119-120°. After that, the 1,2,4-triazole derivative was alkylated by allyl chloride in ethanol solution of KOH. To the solutionof 3-thiol-4-allyl-5-phenyl-4H-1,2,4-triazole (17.44 g,0.080 mol) in ethanol (50 mL) KOH was added (5.20 g, 0.093 mol).The pH value of the solution was 10. Fresh-distilled allyl chloride(6.30 g, 0.082 mol) was added with a dropping funnel. The reaction mixturewas stirred and heated at 65 °C during 15 h. After cooling tort the precipitate of KCl was filtered off and the residual solution was evaporated. 14.3 g (70%) of the ligand L (3-allylsulfanyl-4-allyl-5-phenyl-4H-1,2,4-triazole) was obtained that melts at 49 °C. Ligand (L): 1H NMR (CD3CN, 500 MHz) δ H 7.68e7.58 (2H, m),7.58e7.47 (3H, m), 6.05e5.86 (2H, m), 5.32e5.16 (2H, m), 5.11 (1H, d, J 10.1 Hz), 4.85 (1H, d, J 17.2 Hz), 4.65e4.54 (2H, m), 3.83 (2H,d, J 7.1 Hz). 13C NMR (CD3CN, 126 MHz) δC 155.8, 150.8, 133.4,132.4, 130.2, 128.9, 128.5, 128.4, 127.4, 118.1, 117.4, 116.9, 46.6, 36.2. IR (Nujol, cm1): 411w, 432w, 475w, 495w, 530m, 563m, 587m,603m, 700vs, 770vs, 855w, 877 m, 920s, 936s, 979m, 996m, 1024m,1075m, 1108w, 1137w, 1160w, 1201s, 1232m, 1257w, 1285w, 1298w,1324m, 1353m, 1371m, 1387m, 1424vs, 1438m, 1460vs, 1475s,1525w, 1580m, 1605m, 1635m, 1651m, 1702m, 1773vw, 1821vw,1847w, 1897w, 1960w, 1980vw, 2024vw, 2341w, 2362w, 2548vw,2612vw, 2711vw, 2853w, 2932m, 2976m, 3010m, 3082m.
References:
Hordiichuk, Oleh R.;Kinzhybalo, Vasyl V.;Goreshnik, Evgeny A.;Slyvka, Yurii I.;Krawczyk, Marta S.;Mys'kiv, Marian G. [Journal of Organometallic Chemistry,2017,vol. 838,p. 1 - 8]