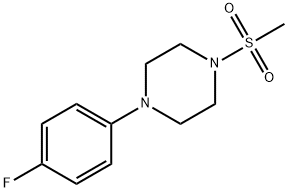
(E)-1-(4-fluorophenyl)-4-(styrylsulfonyl)piperazine synthesis
- Product Name:(E)-1-(4-fluorophenyl)-4-(styrylsulfonyl)piperazine
- CAS Number:260441-13-2
- Molecular formula:C18H19FN2O2S
- Molecular Weight:346.42
Yield:260441-13-2 46%
Reaction Conditions:
Stage #1: with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78;
Stage #2: N-(4-fluorophenyl)-N'-(methanesulphonyl)-piperazine in tetrahydrofuran;hexane at -78; for 1 h;
Stage #3: benzaldehydewith diethyl chlorophosphatemore than 3 stages;
Steps:
1.ii
ii) To a solution of LDA [8.5 mmol; prepared by slow addition of n-butyl lithium (3.5 ml, 8.5 mmol, 2.5 M in hexane) to a solution of diisopropylamine (860 mg, 8.5 mmol) in dry THF (5 ml) at -78° C.] at -78° C. was added a solution of 1-(4-fluorophenyl)4-(methanesulfonyl)piperazine (1 g, 3.87 mmol) in THF (25 ml) dropwise.. The mixture was stirred at -78° C. for 1 hour and a solution of diethylchlorophosphate (670 mg; 3.87 mmol) in THF (3 ml) was added.. The mixture was stirred at -78° C. for 1 hour and benzaldehyde (450 mg; 4.24 mmol) in THF (3 ml) was added.. The mixture was gently warmed to room temperature and stirred for 18 hours.. The mixture was washed with aqueous ammonium chloride and extracted with ethyl acetate.. The organic layers were washed with water, brine and dried over MgSO4.. Purification of the residue on silica (eluant: dichloromethane) afforded 1-(4-fluorophenyl)4(trans-β-styrenesulfonyl)piperazine as a white powder (621 mg, 46%). 1H NMR (CDCl3): 7.50 (m, 3H), 7.43 (m, 3H), 6.97 (m, 2H), 6.89 (m, 2H), 6.71 (d, 1H, J=15.4 Hz), 3.37 (m, 4H), 3.19 (m, 4H).
References:
US6734184,2004,B1 Location in patent:Page/Page column 21; 22
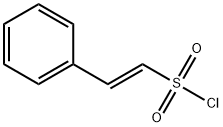
52147-97-4
52 suppliers
$15.00/100mg
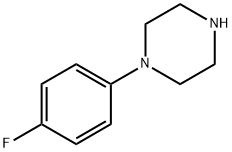
2252-63-3
207 suppliers
$13.43/250mgs:

260441-13-2
4 suppliers
inquiry