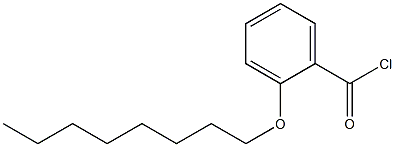
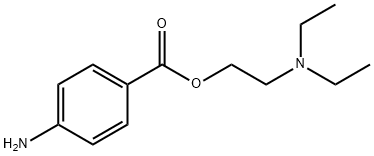
N-DIETHYLAMINOETHYL-P-[2-(-N-OCTYLOXY)-BENZOYL]AMINOBENZOATE synthesis
- Product Name:N-DIETHYLAMINOETHYL-P-[2-(-N-OCTYLOXY)-BENZOYL]AMINOBENZOATE
- CAS Number:26090-29-9
- Molecular formula:C28H40N2O4
- Molecular Weight:468.63
Yield:26090-29-9 500 mg
Reaction Conditions:
with triethylamine in dichloromethane;Inert atmosphere;
Steps:
1.E
The reaction mixture was concentrated to remove residual amount of HCl generated from the reaction, and the reaction was dried under high-vac for 30 minutes before it was added in a solution of dry DCM (5 ml) to a separate reaction flask containing a solution of 8 (315 mg, 1.33 mmol, 0.67 equiv) in anhydrous DCM (5 ml) and Et3N (404 mg, 4 mmol). The reaction was diluted with saturated NaHCO3 solution (20 ml) and extracted with 2-propanol/chloroform (v:v = 1:4) until full recovery of the desired product based on TLC analysis (10% 1M NH3/MeOH in DCM). The combined extracts were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude material was purified by Biotage flash chromatography (gradient elution, 0 to 10% MeOH in DCM) to obtain the title compound 9 (500 mg, 80% yield) as yellow oil.1H NMR (400 MHz, DMSO-d6) d 10.41 (s, 1H), 7.93 (d, J = 8.6 Hz, 2H), 7.84 (d, J = 8.6 Hz, 2H), 7.63 (dd, J = 7.6, 1.8 Hz, 1H), 7.50 (ddd, J = 8.2, 8.2, 1.8 Hz, 1H), 7.17 (d, J = 8.4 Hz, 1H), 7.06 (dd, J = 7.4, 7.4 Hz, 1H), 4.28 (t, J = 6.2 Hz, 2H), 4.09 (t, J = 6.2 Hz, 2H), 2.75 (t, J = 6.0 Hz, 2H), 2.55 (q, J = 7.2 Hz, 4H), 1.74 (m, 2H), 1.38 (m, 2H), 1.31- 1.09 (m, 8H), 0.97 (t, J = 7.2 Hz, 6H), 0.79 (t, J = 6.8 Hz, 3H). LCMS (m/z) 469.4 (M+H); RT (Method B, std LCMS method), 1.10 min.
References:
WO2020/163479,2020,A1 Location in patent:Paragraph 0119