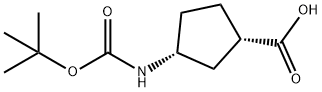
(+)-(1S,3R)-N-BOC-3-AMINOCYCLOPENTANECARBOXYLIC ACID synthesis
- Product Name:(+)-(1S,3R)-N-BOC-3-AMINOCYCLOPENTANECARBOXYLIC ACID
- CAS Number:261165-05-3
- Molecular formula:C11H19NO4
- Molecular Weight:229.27

24424-99-5
850 suppliers
$13.50/25G
71830-07-4
120 suppliers
$55.00/100 mg

261165-05-3
152 suppliers
$39.00/250mg
Yield: 99%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in 1,4-dioxane;water at 20 - 27; for 3 h;
Steps:
21 Typical Procedure for the Preparation of 1,2,4-Ox- adiazoles as Exemplified by the Preparation ofIntermediate 126, (1 R,35)-3-(3-methyl-1 ,2,4-oxadi- azol-5-yl)cyclopentanamine hydrochloride salt
di-tert-l3utyl dicarbonate (1.25 g, 5.75 mmol) and DIPEA (2.61 mE, 15.0 mmol) were added to a solution of (1 S,3R)-3-aminocyclopentanecarboxylic acid (0.646 g, 5.0 mmol) in 1,4-dioxane (5 mE) and water (5 mE) and the resulting mixture was stirred at RT for 3 h. The reaction mixture was acidified to pH 2 using 1 M aqueous HC1 and extracted with DCM (x4). The combined organic extractswere passed through a phase separator cartridge and con-centrated in-vacuo to give (1 S,3R)-3-[(tert-butoxycarbonyl) amino]cyclopentanecarboxylic acid (1.13 g, 99%). ‘H NMR (400 MHz, CDC13) ö: 1.44 (s, 9H),1.56-2.06 (m, 5H), 2.16-2.33 (m, 1H), 2.79-2.93 (m, 1H),3.87-4.18 (m, 1H), 4.86 (bt s., 1H). One exchangeable proton not observed.
References:
Heptares Therapeutics Limited;Brown, Giles Albert;Congreve, Miles Stuart;Pickworth, Mark;Tehan, Benjamin Gerald US2018/105491, 2018, A1 Location in patent:Paragraph 0567; 0773; 0774; 0775; 0776
151907-80-1
165 suppliers
$22.00/250mg

261165-05-3
152 suppliers
$39.00/250mg
![(1S,3R)-Methyl 3-[(tert-butoxycarbonyl)-amino]cyclopentanecarboxylate](/CAS/GIF/554451-12-6.gif)
554451-12-6
14 suppliers
inquiry

261165-05-3
152 suppliers
$39.00/250mg
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1R,4S)-](/CAS/20210111/GIF/693245-53-3.gif)
693245-53-3
2 suppliers
inquiry

261165-05-3
152 suppliers
$39.00/250mg
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)
49805-30-3
372 suppliers
$5.00/5g

261165-05-3
152 suppliers
$39.00/250mg