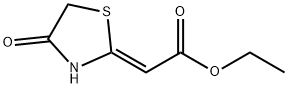
Acetic acid, 2-(4-oxo-2-thiazolidinylidene)-, ethyl ester, (2Z)- synthesis
- Product Name:Acetic acid, 2-(4-oxo-2-thiazolidinylidene)-, ethyl ester, (2Z)-
- CAS Number:26312-94-7
- Molecular formula:C7H9NO3S
- Molecular Weight:187.22
Yield:26312-94-7 95.7%
Reaction Conditions:
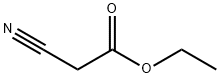
Stage #1: ethyl 2-cyanoacetatewith potassium carbonate at 20; for 0.166667 h;
Stage #2: ethyl 2-sulfanylacetate for 0.833333 h;Reflux;
Steps:
General procedure for the synthesis of 7a-7h
A mixture of ethyl 2-isocyanoacetate (50 mmol) and K2CO3(2.5 mmol) was stirred at room temperature for 10 min. Theethyl 2-mercaptoacetate (52.5 mmol) was then added intothe mixture. The result mixture was heated at reflux for50 min, cooled to the room temperature. Then water and ethanol 73(v/v) (70 mL) were added to the mixture. Then,the mixture was stirred for 1 h and filtrated to afford compound3,9.63 g (yield 95.7%).
References:
Li, Hong-Lian;Li, Wei-Ya;Lu, Xin-Hua;Ma, Ying;Tang, Xue;Wang, Run-Ling;Wu, Jing-Wei;Zhang, Huan;Zheng, Zhi-Hui [Journal of Biomolecular Structure and Dynamics,2020,vol. 38,# 18,p. 5338 - 5348]