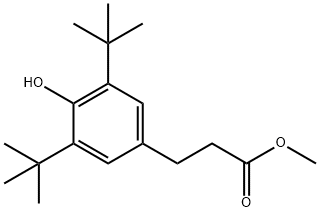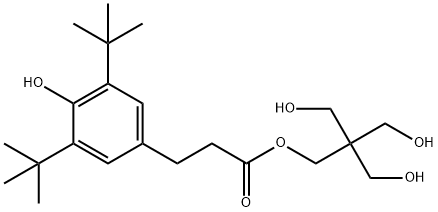
Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, 3-hydroxy-2,2-bis(hydroxymethyl)propyl ester synthesis
- Product Name:Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, 3-hydroxy-2,2-bis(hydroxymethyl)propyl ester
- CAS Number:26347-98-8
- Molecular formula:C22H36O6
- Molecular Weight:396.52
Yield:-
Reaction Conditions:
lithium hydroxide;zinc(II) octanoate at 175; for 3 - 27 h;Product distribution / selectivity;
Steps:
1; 1a; 1b
PP Base (277g, 15% excess), pentaerythritol (28g) and lithium hydroxide monohydrate (0. 15g, 1. 7mol%/Penta) were charged to 500ml litre 4-necked flask equipped with a stirrer, thermometer, pump inlet and distillation head fitted to a condenser, which in turn goes to a liquid-liquid exchanger containing water and cyclohexane. A pump is used to circulate the cyclohexane from the exchanger back into the reactor at a rate of about 0.4g cyclohexane/PP Base g per hour, thus allowing continual distillation of cyclohexane + by-product methanol (the latter being extracted into water by the exchanger). The reaction mass was heated to 175°C with distillation of cyclohexane and methanol. Two shots of zinc octanoate (0.448g, 0. 6mol%/Penta) were added after 5 and 12h. Samples were taken at regular intervals and analysed by HPLC, with results given in area% in the Table below. Time (hrs) PP Acid Disub Tris Anox 20 PP Base 3 0. 4 7. 9 31. 2 27. 3 32. 7 5 0. 4 5. 0 27. 5 38. 6 28. 0 7 0. 4 1. 9 19. 7 56. 2 21. 5 9 0. 4 0. 9 13. 1 66. 8 18. 4 12oui0. 6 7. 8 74. 2 16. 1 14 0_5 03 4. 9 78. 8 15. 0 16 0_5 0. 3 3. 7 80. 2 14. 7 After purification (dilution with cyclohexane, washing, separation of the aqueous layer, drying and crystallisation from cyclohexane), the colour of the Anox 20 final product was found to be APHA 6. Example la Example 1 was repeated but with a reduced excess of PP Base (260g, 8% excess), and with just one shot of zinc octanoate (0.9g, 1. 2mol%/Penta) after 5h. The results are provided in the Table below. Time (hrs) PP Acid Disub Tris Anox 20 PP Base 3 0.4 5.8 29.5 39.4 24.0 5 0. 4 4. 7 27. 2 45. 4 21. 5 7 0.4 2. 2 22. 0 56. 8 17. 6 9 0.4 1. 4 16. 9 65. 1 15. 5 12 0. 5 0. 8 11. 7 73. 5 12. 7 14 0. 6 0. 5 8. 5 77. 9 11. 6 19 0. 7 0. 3 5 80. 0 10. 9 After purification, the colour of the Anox 20 final product was found to be APHA 5 Comparative Example 1 Example 1 was repeated but with lithium hydroxide monohydrate (0.44g, 5mol%/Penta) dosed in eight 0. 055g shots at 2h intervals. The time taken to reach a level of 5% Tris (the point at which the reaction is regarded as complete, and ready to go into the purification stage) was found to be 21h. After purification, the colour of the Anox 20 final product was found to be APHA 14 Comparative Example la Comparative Example 1 was repeated but with lithium hydroxide monohydrate (0.44g, 5mol%/Penta) added all at the beginning of the reaction. The time taken to reach a level of 5% Tris was found to be 28h. After purification, the colour of the Anox 20 final product was found to be APHA 20 Comparative Example lb Comparative example lwas repeated but with lithium hydroxide monohydrate (0. 15g, 1.7 mol%/Penta) and zinc octanoate (0.9g, 1. 2mol%/Penta) added together at the start. The time taken to reach a level of 5% Tris was found to be 27h. After purification, the colour of the Anox 20 final product was found to be APHA 16 These results are summarised in the following Table to illustrate the benefits of the inventive catalyst system
References:
GREAT LAKES CHEMICAL (EUROPE) GMBH WO2005/42463, 2005, A1 Location in patent:Page/Page column 4-6