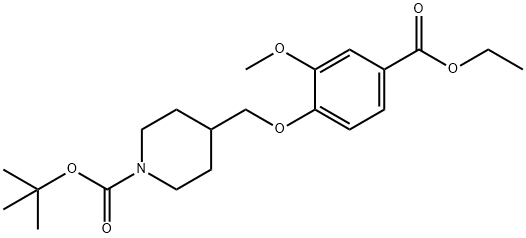
tert-butyl 4-((4-(ethoxycarbonyl)-2-Methoxyphenoxy)Methyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-((4-(ethoxycarbonyl)-2-Methoxyphenoxy)Methyl)piperidine-1-carboxylate
- CAS Number:264208-58-4
- Molecular formula:C21H31NO6
- Molecular Weight:393.4739

617-05-0
185 suppliers
$40.00/20mg
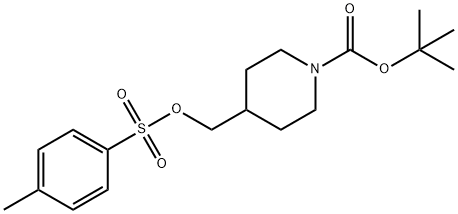
166815-96-9
167 suppliers
$7.00/250mg

264208-58-4
8 suppliers
inquiry
Yield:264208-58-4 89%
Reaction Conditions:
with potassium carbonate in N-methyl-acetamide;
Steps:
9.d Preparation of Compound 9 in Table 2
d) 4-(4-Methylphenylsulphonyloxymethyl)-1-tert-butyloxycarbonylpiperidine (40 g, 0.11 mol) was added to a suspension of ethyl 3-methoxy-4-hydroxybenzoate (19.6 g, 0.1 mol) and potassium carbonate (28 g, 0.2 mol) in dry dimethylformamide (200 ml) and the reaction was heated at 95° C. for 2.5 hours. The reaction was cooled to ambient temperature, partitioned between water and ethyl acetate/diethyl ether, before the organic layer was washed with water and brine. Solvent evaporation in vacuo afforded a clear oil which crystallized on standing. Washing with isohexane and drying in vacuo yielded ethyl 3-methoxy-4-(1-tert-butyloxycarbonylpiperidin-4-ylmethoxy)benzoate (35 g, 89%) as a white solid: m.p. 81-83° C.: 1H NMR (CDCl3): 7.65 (d, 1H), 7.55 (s, 1H), 6.85 (d, 1H), 4.35 (q, 2H), 4.05-4.25 (s, 2H), 3.95 (s, 3H), 3.9 (d, 2H), 2.75 (t, 2H), 2.00-2.15 (m, 2H), 1.80-1.90 (d, 2H), 1.48 (s, 9H), 1.40 (t, 3H), 1.20-1.35 (m, 2H): MS (+ve ESI): 416 (M+Na)30 :
References:
US7235559,2007,B1