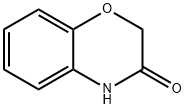
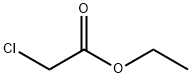
ETHYL 2-(2,3-DIHYDRO-3-OXOBENZO[B][1,4]OXAZIN-4-YL)ACETATE synthesis
- Product Name:ETHYL 2-(2,3-DIHYDRO-3-OXOBENZO[B][1,4]OXAZIN-4-YL)ACETATE
- CAS Number:26673-71-2
- Molecular formula:C12H13NO4
- Molecular Weight:235.24
Yield:26673-71-2 84%
Reaction Conditions:
with potassium carbonate in acetone at 60; for 2 h;Solvent;
Steps:
Methods and procedures for the synthesis of ethyl(3-oxo-2,3-dihydro-4H-1,4-benzoxazin-4-yl)acetate (2)
The ethyl (3-oxo-2,3-dihydro-4H-1,4-benzoxazin-4-yl)acetate(2) has been previously reported. We have prepared andcompared this compound from 2H-benzo[1,4]oxazin-3(4H)-one (1) and ethyl bromoacetate in two different conditions.The obtained compound was then recrystalized from ethanolto give the desired product. The melting points as well asspectroscopic data of this ethyl ester derivative are inaccordance with published data.33b Method A: A mixture of benzoxazin-3(4H)-one (2g,13 mmol) and ethyl bromoacetate (3.34g, 20 mmol) in thepresence of anhydrous K2CO3 (3.59g, 26 mmol) was dissolvedin dry acetone and was stirred at 60°C for 2h. The progress ofthe reaction was monitored by TLC. After the completions ofthe reaction, the reaction mixture was filtered and the filtrateevaporated under reduced pressure. The residue obtained was recrystallized from ethanol to give the suitable compound as awhite solid.Method B: Compound (2) was synthesized in the similarmanner by a mixture of benzoxazin-3(4H)-one (2g, 13 mmol),ethyl bromoacetate (2.67g, 16. mmol), K2CO3 (0.10g,14.37 mmol) in DMF as solvent, and the reaction was stirredat 100°C for 2h. At the end of this period, the mixture wasdiluted with water. The separated solid formed was filtered,washed repeatedly with water and recrystallized from ethanolto give the desired compound, which is in any point identicalto that found by method A.Compounds 2 (method A and method B) was obtained aswhite solid. Yield: method A (84%) and method B (82%); Mp81-82°C. IR (KBr, cm-1): 1738, 2915, 2983. 1H-NMR (400MHz, DMSO-d6): δ = 1.26-1.29 (t, 3H, CH2-CH3, CH3), 4.21-4.27 (q, 2H, -CH2-CH3, CH2) 4.65-4.68 (d, 4H, CH2COOEt,-NCO-CH2O-, 2CH2), 6.74-7.26 (m, 4H, Ar-H). 13C-NMR(100 MHz, CDCl3): 167.91 (C=O), 165.04 (C=O), 145.2,128.76, 124.37, 123.06, 117.33, 114.53 (aromatic carbons),67.62 (CH2), 62.00 (CH2), 43.07 (CH2), 14.27 (CH3).
References:
Bentoumi, Houria;Chettibi, Naouel;Liacha, Messaoud [Revue Roumaine de Chimie,2020,vol. 65,# 10,p. 885 - 891]
95-55-6
528 suppliers
$5.00/5G
![ETHYL 2-(2,3-DIHYDRO-3-OXOBENZO[B][1,4]OXAZIN-4-YL)ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3211317.gif)
26673-71-2
9 suppliers
inquiry
708262-58-2
1 suppliers
inquiry
![ETHYL 2-(2,3-DIHYDRO-3-OXOBENZO[B][1,4]OXAZIN-4-YL)ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3211317.gif)
26673-71-2
9 suppliers
inquiry

10147-68-9
17 suppliers
$45.00/50mg
![ETHYL 2-(2,3-DIHYDRO-3-OXOBENZO[B][1,4]OXAZIN-4-YL)ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3211317.gif)
26673-71-2
9 suppliers
inquiry