
2-METHOXY-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PHENOL synthesis
- Product Name:2-METHOXY-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PHENOL
- CAS Number:269410-22-2
- Molecular formula:C13H19BO4
- Molecular Weight:250.1
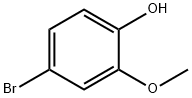
7368-78-7
189 suppliers
$24.00/1g
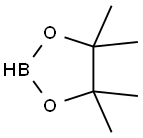
25015-63-8
217 suppliers
$22.39/5G

269410-22-2
108 suppliers
$21.00/250mg
Yield:269410-22-2 62 %Chromat.
Reaction Conditions:
Stage #1: with triethylamine;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 80;
Stage #2:4-bromoguaiacol;4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane with triethylamine in 1,4-dioxane at 100; for 20 h;
Steps:
74 2-Methoxy-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-phenol
[00413] Solution 1: Pinacol (124.6 g; 1.054 mol) in dry dioxane (200 ml) was stirred under N2 and allowed to equilibrate to RT. [00414] Solution 2: Borane-methyl sulphide complex (100 ml; 10M; 1.00 mol) in dioxane (250 ml) was stirred at RT under N2. [00415] Solution 3: PdCl2(dppf)CH2Cl2 (7.27 g; 8.90 mmol; 2 mole %) and NEt3 (25.0 ml; 0.179 mol) in dry dioxane (300 ml) was stirred at 80 C., under N2, overnight before cooling to room temperature. [00416] Solution 1 was transferred under N2 to a pressure-equalising dropping funnel and added to Solution 2 over 6 h. Stirred at RT overnight before heating at 40-50 C. for 8 h. After stirring overnight at RT a white ppt had formed. NEt3 (149 ml; 1.07 mol) was added followed by slow addition of 2-methoxy-4-bromophenol (91.6 g; 0.451 mol). Finally, the activated catalyst (Solution 3) was added and the resultant dark brown solution was stirred at 100 C. GC analysis after 20 hours showed the desired borate compound at 11.1 minutes (62%).
References:
Commonwealth Scientific and Industrial Research Organisation US6680401, 2004, B1 Location in patent:Page column 66

7368-78-7
189 suppliers
$24.00/1g

73183-34-3
563 suppliers
$6.00/5g

269410-22-2
108 suppliers
$21.00/250mg

54771-60-7
66 suppliers
$35.00/1g

269410-22-2
108 suppliers
$21.00/250mg
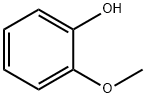
90-05-1
735 suppliers
$5.00/1g

269410-22-2
108 suppliers
$21.00/250mg