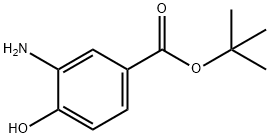
Benzoic acid, 3-amino-4-hydroxy-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Benzoic acid, 3-amino-4-hydroxy-, 1,1-dimethylethyl ester (9CI)
- CAS Number:273939-23-4
- Molecular formula:C11H15NO3
- Molecular Weight:209.24
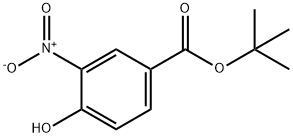
273939-22-3
11 suppliers
$276.14/250mg

273939-23-4
11 suppliers
inquiry
Yield:273939-23-4 95%
Reaction Conditions:
palladium-carbon in methanol;
Steps:
4 tert-butyl 4-hydroxy-3-aminobenzoate
tert-butyl 4-hydroxy-3-aminobenzoate To a solution of tert-butyl 4-hydroxy-3-nitrobenzoate (4.4 g, 18.4 mmol) in methanol (50 mL), 10% Pd/C (200 mg) was added and the resulting suspension was hydrogenated at 50 psi for 4 hours. After filtering under a pad of celite, methanol was removed in vacuo to obtain the title compound as a greenish powder (3.5 g, 95%). MS: m/z 210 (M+H)+, 419 (2M+H)+, 208 (M-H)- 1H-NMR (300 MHz), δ(CDCl3): 1.7 (s, 9H), 4.2 (broad singlet, 2H) 6.75 (d, 1H), 7.6 (m, 2H).
References:
US2003/69236,2003,A1