1,2:3,4-DI-O-ISOPROPYLIDENE-ALPHA-L-ARABINOPYRANOSE synthesis
- Product Name:1,2:3,4-DI-O-ISOPROPYLIDENE-ALPHA-L-ARABINOPYRANOSE
- CAS Number:27820-98-0
- Molecular formula:C11H18O5
- Molecular Weight:230.26
Yield:27820-98-0 95%
Reaction Conditions:
with sulfonated polystyrene cation exchange resin CAT600 at 40; for 3 h;Green chemistry;
Steps:
General Procedure for the O-Isopropylidenation.
General procedure: To asuspension of the substrate (2 mmol) in dry acetone (10 mL),CAT600 (100 mg) was added. Then the mixture was stirred at 40 °C till the TLC (n-hexane-EtOAc 2:1) showed the completion of the reaction. The catalyst was separated by filtration, washed with acetone, dried, and reused for a consecutive run under the same reaction conditions. The filtrate was condensed to dry in vacuum, and the residue was dissolved in CH2Cl2 (10 mL) and washed with 3 × 5 mL brine.The organic layer was dried over anhydrous Na2SO4, filtered and evaporated to afford the crude product. Then the desired pure product was obtained by recrystallization from n-hexane. While, the silica gel column chromatography was used if the product existed in the liquid form.
References:
Rong, Yuan Wei;Zhang, Qi Hua;Wang, Wei;Li, Bao Lin [Bulletin of the Korean Chemical Society,2014,vol. 35,# 7,p. 2165 - 2168]
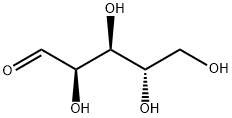
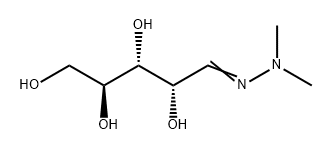
5328-37-0
458 suppliers
$5.00/1g

67-64-1
6 suppliers
$21.60/10ml
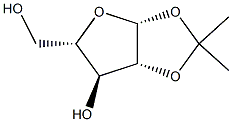
51885-64-4
4 suppliers
inquiry

27820-98-0
21 suppliers
inquiry

5328-37-0
458 suppliers
$5.00/1g

27820-98-0
21 suppliers
inquiry