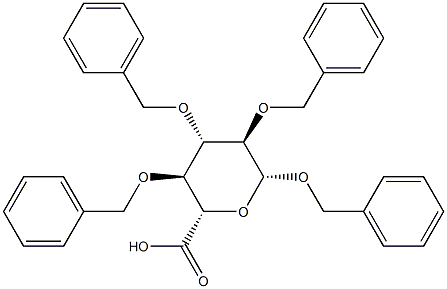
Benzyl 2-O,3-O,4-O-tribenzyl-β-D-glucopyranosiduronic acid synthesis
- Product Name:Benzyl 2-O,3-O,4-O-tribenzyl-β-D-glucopyranosiduronic acid
- CAS Number:27851-26-9
- Molecular formula:C34H34O7
- Molecular Weight:554.63
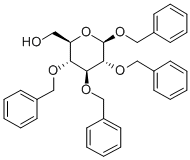
27851-29-2
61 suppliers
$44.79/5MG

27851-26-9
1 suppliers
inquiry
Yield:27851-26-9 0.44 g
Reaction Conditions:
with potassium dichromate;sulfuric acid in acetone at 55; for 2 h;
Steps:
3.6 Step 6: (2S,3S,4S, 5R, 6R)-3 ,4, 5 ,6-Tetrakis(benzyloxy)tetrahydro-2H-pyran-2-carboxylic acid
((2R,3R,4S,5R,6R)-3 ,4,5 ,6-Tetrakis(benzyloxy)tetrahydro-2H-pyran-2-yl)methanol (0.67 g, 1.24 mmol) was dissolved in acetone (30 mL) and a solution of potassium dichromate (0.62 g, 1.24 mmol) in 6M sulphuric acid (9 mL) was added. The reaction mixture was heated at 55 °C for 2 hours. The reaction was diluted with water (300 mL) and extracted with DCM (3 x 100 mL). The combined organic layers were dried (Mg504) and concentrated. The product was purified by flash column chromatography (5i02, eluting with acetone/petroleum ether 3:7 v/v) to give (2S,3S,4S,5R,6R)-3 ,4,5 , 6-tetrakis(benzyloxy)tetrahydro-2H-pyran-2-carboxylic acid (0.44 g). [1571 ‘H NMR (CDC13): ? 7.45-7.10 (m, 20H), 4.95-4.43 (m, 9H), 3.92 (d, 1H), 3.76 (dd, 1H),3.62 (dd, 1H), 3.46 (dd, 1H).
References:
WO2014/2039,2014,A1 Location in patent:Paragraph 156; 157

66966-07-2
16 suppliers
$165.00/50mg

27851-26-9
1 suppliers
inquiry
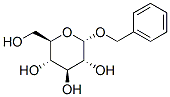
4304-12-5
68 suppliers
$125.00/250mg

27851-26-9
1 suppliers
inquiry

100-51-6
1383 suppliers
$5.00/100g

27851-26-9
1 suppliers
inquiry