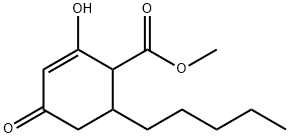
methyl 6-n-pentyl-2-hydroxy-4-oxo-cyclohex-2-ene-1-carboxylate synthesis
- Product Name:methyl 6-n-pentyl-2-hydroxy-4-oxo-cyclohex-2-ene-1-carboxylate
- CAS Number:27871-89-2
- Molecular formula:C13H20O4
- Molecular Weight:240.3
Yield:27871-89-2 94 g
Reaction Conditions:
with sodium methylate in methanol; for 3 h;Inert atmosphere;Reflux;
Steps:
B.1 1. Methyl 6-N-Pentyl-2-hydroxy-4-oxo-cyclohex-2-ene-l-carboxylate
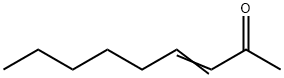
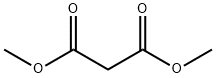
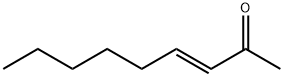
[0119] To a stirring solution of sodium methoxide (32.4 g, 0.60 mol) and dimethyl malonate (90 g, 0.68 mol) in 230 mL of anhydrous methanol was added portion wise 75 g (0.48 mol) of 90% 3-nonen-2-one. The reaction mixture was then refluxed for 3 h under N2 and allowed to cool to room temperature. The solvent was distilled under reduced pressure and the residue dissolved in 350 mL of water. The slurry of white crystals and the almost clear solution was extracted thrice with 80 mL of chloroform. The aqueous layer was acidified to pH 4 with concentrated HCl and the white precipitate that formed was allowed to stand overnight prior to filtration. The crystals were dried at 50° C under high vacuum for 5 hours to yield 106.5 g (0.4416 mol) (92%) of methyl 6-n-Pentyl-2-hydroxy-4-oxo-cyclohex-2-ene-l-carboxylate (mp 96-98 C). The product was recrystallized using a mixture of petroleum ethenethyl acetate (9: 1), and gave 94 g of pure methyl 6-n-Pentyl-2-hydroxy-4-oxo-cyclohex-2-ene-l-carboxylate (melting point of 98-100 C).
References:
WO2014/134281,2014,A1 Location in patent:Paragraph 0119