
Benzeneacetic acid, .alpha.-broMo-2-chloro-, ethyl ester synthesis
- Product Name:Benzeneacetic acid, .alpha.-broMo-2-chloro-, ethyl ester
- CAS Number:2856-79-3
- Molecular formula:C10H10BrClO2
- Molecular Weight:277.5422

40061-54-9
73 suppliers
$35.75/25g

2856-79-3
14 suppliers
inquiry
Yield:2856-79-3 100%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane; for 8 h;Heating / reflux;
Steps:
6
Ethyl 2-(2-chlorophenyl)-2-(1H-imidazol-1-yl)acetate (26)A mixture of ethyl 2-chlorophenylacetate (2.3 g, 11.60 mmol), N- bromosuccinimmide (2.3 g, 12.81 mmol) and a catalytic amount of α,α'- azobis(isobutyronitrile) in carbon tetrachloride (27.0 ml_) was heated under reflux for 8h. The reaction mixture was cooled to room temperature and the resulting suspension was filtered over cotton. The organic filtrate was washed with 1 N solution of NaOH (3 x 5 ml_), dried over Na2SO4 and the solvent was removed. The ethyl 2-bromo-2-(2-chlorophenyl)acetate (3.2 g, 100%) thus obtained was dissolved in acetone (40 ml_) in the presence of imidazole sodium derivative (1.04
References:
WO2007/104696,2007,A1 Location in patent:Page/Page column 61-62
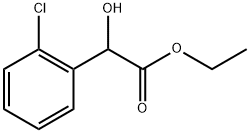
13511-30-3
1 suppliers
inquiry

2856-79-3
14 suppliers
inquiry
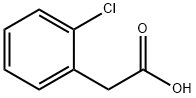
2444-36-2
445 suppliers
$5.00/25g

2856-79-3
14 suppliers
inquiry
![Benzeneacetonitrile, 2-chloro-α-[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/66985-47-5.gif)
66985-47-5
0 suppliers
inquiry

2856-79-3
14 suppliers
inquiry

89-98-5
662 suppliers
$15.00/25g

2856-79-3
14 suppliers
inquiry