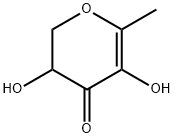
2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one synthesis
- Product Name:2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one
- CAS Number:28564-83-2
- Molecular formula:C6H8O4
- Molecular Weight:144.13
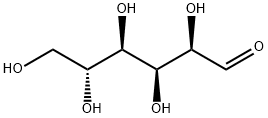
50-99-7
986 suppliers
$6.00/25g

28564-83-2
50 suppliers
inquiry
Yield:28564-83-2 16.5%
Reaction Conditions:
Stage #1:D-glucose with pyrrolidine in ethanol at 60; for 4 h;
Stage #2: with acetic acid in ethanol at 75; for 40 h;
Steps:
1; 4 Embodiment 1
Preparation of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one: in a 500 mL three-necked round bottom flaskIn the process, add 36g of D-glucose (0.2mol), 28g of tetrahydropyrrole (0.4mol) and 250mL of absolute ethanol.In a blender, stir at 60C for 4h until it is clear. Then dissolve 24g of acetic acid (0.4mol) in 50mL of absolute ethanol and place it in a constant pressure dropping funnel.Slowly dropwise into the three-necked flask, and the temperature was raised to 75 ° C. and the reaction was continued for 40 h. After the reaction, cool to room temperature, 45C, 75Pa conditionsAfter the solvent was distilled off under reduced pressure, a viscous Maillard reaction product was obtained.Using silica gel chromatography column-polyamide resin column-silica gel column chromatography three times for viscous Maillard reaction productsIsolation and purification. First, dissolve the Maillard reaction product with methanol, add silica gel (100 ~ 200 mesh), mix well and reduce the pressureEvaporate the solvent to loose sand, and add it to the first silica gel column (200 ~ 300 mesh silica gel) as a dry sample. DichloromethaneMethane / methanol = 50: 1 mixed solvent is used as the eluent. The eluate is detected by the detector. When it contains 2,3-dihydro-3,5-dihydroxy 6-methyl-4H-pyran-4-one When the crude product flows out, the detector introduces the effluent to the inlet of the transfer pump and passes through the transfer pump.Conveyed to the first-stage concentration device at the upper end of the polyamide resin column. The first stage concentration device receives a certain volume of effluentAfter that, the stirring heating device was started, concentrated to a certain volume, and then flowed into the polyamide resin column. Similarly, the crude product is polyamideAfter the resin column and the second silica gel column, a pure 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one product was obtained.The total yield was 16.54%. After nuclear magnetic resonance and GC-MS detection, it was confirmed to be the target with a purity of more than 98%. (Of which polyamideThe resin column was mixed with petroleum ether / acetone = 7: 1 as the eluent, and the second silica gel column was mixed with dichloromethane / methanol = 50: 1.The combined solvent is the eluent.
References:
Henan China National Tobacco Industry Co., Ltd.;Chen Zhifei;Xi Gaolei;Xu Kejing;Zhao Zhiwei;Cai Lili;Sun Zhitao;Yang Jing;Ma Shengtao;Liu Qiang CN110818665, 2020, A Location in patent:Paragraph 0036-0038; 0045-0049

59-23-4
633 suppliers
$6.00/25g

28564-83-2
50 suppliers
inquiry
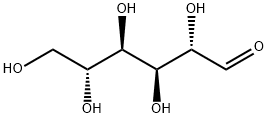
3458-28-4
535 suppliers
$10.00/5g

28564-83-2
50 suppliers
inquiry

28787-36-2
31 suppliers
inquiry

28564-83-2
50 suppliers
inquiry
