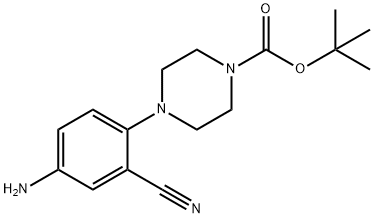
tert-Butyl 4-(4-aMino-2-cyanophenyl)piperazine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-aMino-2-cyanophenyl)piperazine-1-carboxylate
- CAS Number:288251-85-4
- Molecular formula:C16H22N4O2
- Molecular Weight:302.37
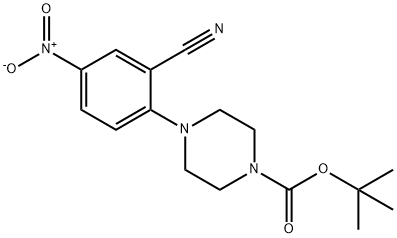
288251-87-6
33 suppliers
inquiry

288251-85-4
36 suppliers
inquiry
Yield:288251-85-4 88.5%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 90; for 1 h;
Steps:
S55.2 Step-2: Synthesis of tert-butyl 4-(4-amino-2-cyanophenyl)piperazine-1-carboxylate
To a stirred solution of 567 tert-butyl 4-(2-cyano-4-nitrophenyl)piperazine-1-carboxylate (2.0 g, 6.021 mmol, 1.0 eq) in 6 EtOH (30 mL) was added Fe(0) (1.0 g, 18.064 mmol, 3 eq) and a solution of 67 NH4Cl (644 mg, 12.042 mmol, 2 eq) in 7 water (30 mL) at rt. The resulting mixture was heated at 90° C. for 1 hr. The progress of reaction was monitored by LCMS. The reaction mixture was filtered through celite the residue was washed with EtOH (50 mL). The filtrate was concentrated and the residue was dissolved in 19 EtOAc (100 mL), washed with water (2×100 mL), dried over Na2SO4, and concentrated to afford the desired compound, 570 tert-butyl 4-(4-amino-2-cyanophenyl)piperazine-1-carboxylate (1.77 g, 88.5%). (0598) LCMS: 303.2 [M+1]+
References:
US2019/106436,2019,A1 Location in patent:Paragraph 0594; 0597-0598
17417-09-3
232 suppliers
$9.00/1g

288251-85-4
36 suppliers
inquiry

57260-71-6
740 suppliers
$5.00/5g

288251-85-4
36 suppliers
inquiry

16588-15-1
44 suppliers
$60.00/50mg

288251-85-4
36 suppliers
inquiry
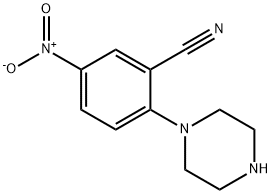
288251-86-5
4 suppliers
inquiry

288251-85-4
36 suppliers
inquiry