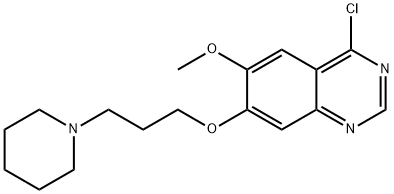
4-Chloro-6-methoxy-7-(3-piperidin-1-yl-propoxy)-quinazoline synthesis
- Product Name:4-Chloro-6-methoxy-7-(3-piperidin-1-yl-propoxy)-quinazoline
- CAS Number:288383-71-1
- Molecular formula:C17H22ClN3O2
- Molecular Weight:335.83
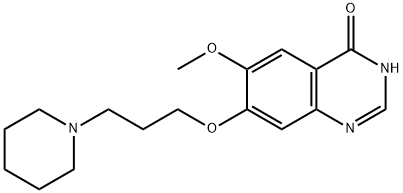
288383-74-4
4 suppliers
inquiry

288383-71-1
23 suppliers
inquiry
Yield: 73%
Reaction Conditions:
Stage #1:6-methoxy-7-(3-(piperidin-1-yl)propoxy)quinazolin-4-ol with trichlorophosphate in toluene;acetonitrile at 42;Industry scale;
Stage #2: with ammonium hydroxide in toluene;acetonitrile at 35 - 57;Industry scale;
Steps:
4
6-Methoxy-7-(3-(piperidin-l-yl)propoxy)quinazolin-4-ol (VI) (17.2 kg, 54.2 mol) was added to toluene (88.1 kg), then mixed with acetonitrile (20.5 kg) and N,N'-diisopropylethylamine (8.9 kg, 68.9 mol) before a controlled addition of phosphorous oxychloride (12.3 kg, 80.2 mol) over 9 minutes. The reaction solution was heated to 42 0C and stirred for 19 hours until the reaction was deemed complete by HPLC. The product solution was quenched by adding an ammonium hydroxide solution over 39 minutes. After the completion of the quench, the solution was at pH 11. The solution was then heated to 35 0C, stirred for 47 minutes, and heated further to 57 0C. The aqueous layer was removed and the organics were washed twice with RO/DI water. During the second water wash, an emulsion occurred. The emulsion layer was combined with the remaining organics and the two water washes were repeated. Lastly, the organics were washed with a saturated brine solution before undergoing a removal of toluene by an azeotropic distillation with additional acetonitrile (121.4 kg). The product was crystallized by first heating to 78 °C for complete dissolution, then cooling to 52 0C, stirring for 1 hour 40 minutes, cooling to 25 0C, stirring for 2 hours, cooling to 5°C, and finally, stirring for 2 hours 25 minutes. The solids were isolated, washed twice with cold acetonitrile (4.5 0C), and dried at < 40 0C for 41 hours 40 minutes. The product was discharged, yielding 13.2 kg of the title compound (VII) (73% yield, 98.7% purity HPLC AUC). 1H ΝMR (300 MHz, d6-DMSO, δ): 7.96 (s, IH), 7.42 (s, IH), 7.13 (s, IH), 4.14 (m, 2H), 3.86 (s, 3H), 2.35 (m, 6H), 1.88 (m, 2H), 1.49 (m, 4H) and 1.30 (m, 2H).
References:
MILLENNIUM PHARMACEUTICALS, INC;ARMITAGE, Ian;BOURLAND, Michael, E.;BOYLE, Craig, J.S.;COOPER, Martin, Ian;FERDOUS, Abu, J.;LANGSTON, Marianne WO2010/59239, 2010, A2 Location in patent:Page/Page column 42-43

288383-74-4
4 suppliers
inquiry

288383-71-1
23 suppliers
inquiry
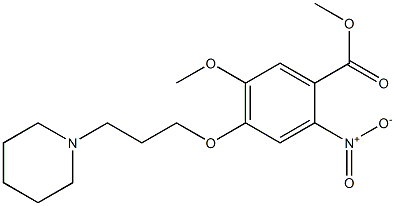
927173-22-6
1 suppliers
inquiry

288383-71-1
23 suppliers
inquiry

3943-74-6
350 suppliers
$5.00/100mg

288383-71-1
23 suppliers
inquiry
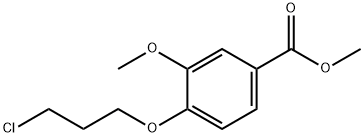
111627-40-8
18 suppliers
inquiry

288383-71-1
23 suppliers
inquiry