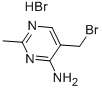
4-Pyrimidinamine, 5-(bromomethyl)-2-methyl-, monohydrobromide synthesis
- Product Name:4-Pyrimidinamine, 5-(bromomethyl)-2-methyl-, monohydrobromide
- CAS Number:2908-71-6
- Molecular formula:C6H9Br2N3
- Molecular Weight:0
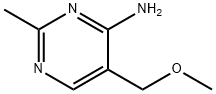
769-82-4

2908-71-6
S4. 100 g of the ring-closing product (cas:769-82-4) was placed in a 1 liter wide-mouth reaction flask and 500 mL of 33% hydrobromic/acetic acid solution was added. The reaction system was rapidly heated to 100 °C and maintained at this temperature for 8-16 h. The endpoint of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, 20 g of acidic activated carbon was added to the reaction mixture and stirred for 30 min before thermal filtration. The filtrate was concentrated to dryness under reduced pressure. Subsequently, 30 mL of anhydrous ethanol was added and filtered after cooling to precipitate crystals. Finally, 1.49 g of 5-(bromomethyl)-2-methylpyrimidin-4-amine hydrobromide was obtained by drying in 87% yield.

769-82-4
7 suppliers
inquiry

2908-71-6
30 suppliers
inquiry
Yield:2908-71-6 87%
Reaction Conditions:
with hydrogen bromide in acetic acid at 100;Temperature;Solvent;
Steps:
3.4 A method for synthesizing 4-amino-2-methyl-5-(bromomethyl)pyrimidine hydrobromide, Include the following steps:
S4. Add 100 grams of ring-closing product to a 1-liter jar.Slowly add 500 ml of 33% HBr/HOAc solution.Slowly warm the system to 100°C,Maintain this temperature for 8-16 hours,TLC determines the endpoint of the reaction.After the reaction is completed, 20 grams of acidic activated carbon is added while hot, and the mixture is stirred for 30 minutes and then filtered. The filtrate is concentrated under pressure and dried to dryness. 30 milliliters of anhydrous ethanol is added, and the crystals are cooled and filtered.149 g of the target product was dried to give a yield of 87%.
References:
CN107382877,2017,A Location in patent:Paragraph 0031; 0032-0086; 0091; 0096; 0101
73-66-5
26 suppliers
inquiry

2908-71-6
30 suppliers
inquiry

749835-80-1
3 suppliers
inquiry

2908-71-6
30 suppliers
inquiry