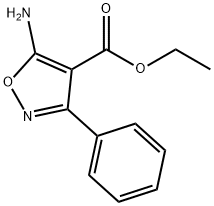
5-Amino-3-phenyl-4-isoxazolecarboxylic acid ethyl ester synthesis
- Product Name:5-Amino-3-phenyl-4-isoxazolecarboxylic acid ethyl ester
- CAS Number:29278-09-9
- Molecular formula:C12H12N2O3
- Molecular Weight:232.24
Yield:-
Reaction Conditions:
Stage #1: benzoyl chloride;ethyl 2-cyanoacetatewith triethylamine at 20; for 48 h;
Stage #2: with triethylamine;trichlorophosphate in dichloromethane;Heating / reflux;
Stage #3: with sodium hydroxide;hydroxylamine hydrochloride in water at 20; for 3 h;
Steps:
Preparation of ethyl 5-amino-3-phenylisoxazole-4-carboxylate; Into a round bottom flask was added benzoyl chloride (2.5 mL, 0.022 mol), benzene (100 mL, 1 mol) and cyanoacetic acid ethyl ester (1.1 mL, 0.011 mol) (prepared according to the procedure of Gilman, H., and A. H. Blatt, Organic Syntheses, 1941, 1, 254-256). Triethylamine (3.0 mL, 0.022 mol) was added and the reaction turned orange and thick. The reaction was stirred at ambient temperature for 48 hours. The reaction was diluted with water and separated. The organic layer was dried over Na2SO4, and filtered and concentrated to leave a orange oil. The oil was purified by flash chromatography on silica gel (gradient 0-40% over 60 min, ethyl acetate/heptane). The fraction containing the correct mass by LCMS was concentrated to dryness, dissolved in DCM (100 mL, 2 mol) and placed into a round bottom flask. To the solution was added phosphoryl chloride (1 mL, 0.01 mol). Triethylamine (3 mL, 0.02 mol) was added dropwise to the solution while stirring. The reaction changed from yellow to dark red upon heating the mixture to reflux. The reaction was refluxed overnight. The reaction was cooled and extracted with HCl (5 M). The solvent was evaporated under reduced pressure to leave an orange/brown tar. The tar was dissolved in ether (50 mL) and washed with HCl (5 M) and sodium bicarbonate solution. The organic phase was collected, dried over Na2SO4, filtered and the solvent was evaporated under reduced pressure. The residue was purified by flash chormatography on silica gel using a 0-50% ethyl acetate/heptane gradiant over 60min to afford a residue to which 1 equivalent of hydroylamine-hydrochloride in ImL of 10% NaOH was added. The reaction was stirred for 3h at ambient temperature. Water was added to the reaction and then it was extracted with DCM (3 x 50 mL). The organic extracts were combined, dried over Na2SO4, filtered and concentrated to dryness. The residue was purified by flash chromatography on silica gel using a 0-40% ethyl acetate/heptane gradiant over 35 min to afford ethyl 5-amino-3-phenylisoxazole-4-carboxylate (498mg, 2 mmol) as a white powder. LCMS (Table 1, Method a) R, = 2.46 min, m/z 233.1 (M+H)+.
References:
WO2009/11850,2009,A2 Location in patent:Page/Page column 154
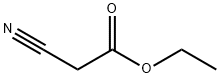
105-56-6
433 suppliers
$10.00/5ml
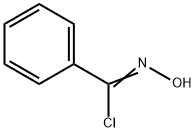
698-16-8
93 suppliers
$16.00/100mg

29278-09-9
18 suppliers
inquiry
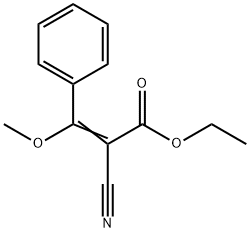
70638-61-8
4 suppliers
inquiry

29278-09-9
18 suppliers
inquiry

698-16-8
93 suppliers
$16.00/100mg

29278-09-9
18 suppliers
inquiry

