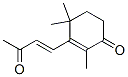
(E)-2,4,4-trimethyl-3-(3-oxo-1-butenyl)cyclohex-2-en-1-one synthesis
- Product Name:(E)-2,4,4-trimethyl-3-(3-oxo-1-butenyl)cyclohex-2-en-1-one
- CAS Number:29790-29-2
- Molecular formula:C13H18O2
- Molecular Weight:206.2808
79-77-6
246 suppliers
$10.00/25g

29790-29-2
5 suppliers
inquiry
Yield:29790-29-2 75%
Reaction Conditions:
with sodium bromate;sodium hydrogen sulfate;potassium iodide in dichloromethane;water at 35 - 40; for 6.5 h;
Steps:
2
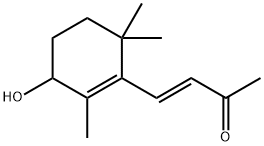
Example 2; 4-oxo-β-ionone 5 IInto a suitable reaction vessel, under an atmosphere of nitrogen, is loaded 100 g of 4-oxo-β-ionone (520 mmol) and 500 mL of methylene chloride; the reaction mixture is heated at 37°C and in the meantime a solution containing 9.6 g of potassium iodide (58 mmol) dissolved in 60 mL of water added, followed by a solution containing 17 g of sodium bisulphate monohydrate (123 mmol) dissolved in 35 mL of water. A
References:
WO2007/72529,2007,A2 Location in patent:Page/Page column 21-22
![4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-3-buten-2-one](/CAS/GIF/23267-57-4.gif)
![3-Buten-2-one, 4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-, (3E)-](/CAS/20200611/GIF/36340-49-5.gif)
