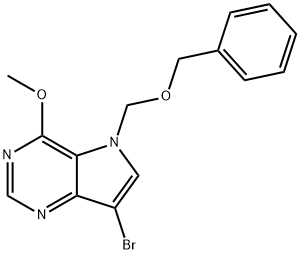
5H-Pyrrolo3,2-dpyrimidine, 7-bromo-4-methoxy-5-(phenylmethoxy)methyl- synthesis
- Product Name:5H-Pyrrolo3,2-dpyrimidine, 7-bromo-4-methoxy-5-(phenylmethoxy)methyl-
- CAS Number:299916-62-4
- Molecular formula:C15H14BrN3O2
- Molecular Weight:348.19
![5-(benzyloxyMethyl)-4-Methoxy-5H-pyrrolo[3,2-d]pyriMidine](/CAS/20150408/GIF/1026012-16-7.gif)
1026012-16-7
5 suppliers
inquiry

299916-62-4
41 suppliers
$403.00/100mg
Yield: 77%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 0 - 20; for 0.5 h;Inert atmosphere;Heating;
Steps:
1.6 Step 6: Preparation of6-Methoxy-N-(benzyloxymethyl)-9-bromo-9-deazahypoxanthine (27f)
To a solution of 6-Methoxy-N-(benzyloxymethyl)-9-deazahypoxanthine (27e)(59 .81 g, 222 mmol) in dichloromethane (225 mL) under N2 cooled to 4 °C (homogenousreaction mixture) was added NBS (40.3 g, 224 mol, 1.01 eq) in portions over 30 min suchthat the reaction temperature remained below 15 °C. The mixture was stirred at 0 °C for 15mins and allowed to warm to room temperature over 15 mins (TLC analysis 50% ethyl acetate in hexane). The reaction mixture was vacuum filtered to remove insolublesuccinimide. The filtrate was washed with water (2 x 250 mL) and brine (200 mL), dried(Na2S04), filtered and concentrated in vacuo to furnish product as a light brown solid. Thesolid was dissolved by boiling in ethyl acetate (200 mL) and diluted with hexane (200 mL).The solution was boiled to reflux and filtered hot very quickly (to avoid solid crystallizing out). The filtrate was then boiled and added hexane in increments of200 mL (total volumeofhexane 1600 mL). The hot solution was decanted if needed to remove insoluble residues(the product is soluble in hot 10% ethyl acetate in hexane). The hot filtrate was allowed tocool to room temperature and then kept in freezer overnight. The solid obtained was collected by filtration and washed with hexane and dried in vacuo at room temperature tofurnish 6-methoxy-N-(benzyloxymethyl)-9-bromo-9-deazahypoxanthine (27f) (59.6 g, 77%), as a light yellow solid: MP 103- 108 °C; 1H NMR (DMSO-d6) 8 8.51 (s, 1H), 8.12 (s,1H), 7.31 -7.22 (m, 5H), 5.74 (s, 2H), 4.52 (s, 2H), 4.07 (s, 3H). 13C-NMR (DMSO-d6) 156.19, 150.66, 148.14, 137.59, 133.45, 128.38, 127.80, 127.67, 115.02, 90.90, 77.79,70.25, 54.07; IR (KBr) 3078, 1602, 1542 cm-1; MS (ES+) 348.27 (100 %), 350.28 (98 %);Analysis: Calculated for C1sH14N30zBr: C, 51.74; H, 4.05; N, 12.07; Found: C, 51.72; H,4.04; N, 12.06.
References:
BIOCRYST PHARMACEUTICALS, INC.;KOTIAN, Pravin, L.;BABU, Yarlagadda, S. WO2013/158746, 2013, A1 Location in patent:Page/Page column 91; 92
![1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/5655-01-6.gif)
5655-01-6
113 suppliers
$45.00/50mg

299916-62-4
41 suppliers
$403.00/100mg
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
198 suppliers
$9.00/250mg

299916-62-4
41 suppliers
$403.00/100mg
180059-04-5
36 suppliers
$45.00/50mg

299916-62-4
41 suppliers
$403.00/100mg
![ethyl 4-hydroxy-5H-pyrrolo[3,2-d]pyriMidine-7-carboxylate](/CAS/20150408/GIF/779326-74-8.gif)
779326-74-8
7 suppliers
inquiry

299916-62-4
41 suppliers
$403.00/100mg