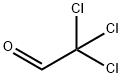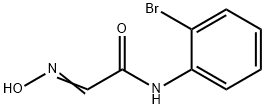
(2E)-N-(2-BROMOPHENYL)-2-(HYDROXYIMINO)ACETAMIDE synthesis
- Product Name:(2E)-N-(2-BROMOPHENYL)-2-(HYDROXYIMINO)ACETAMIDE
- CAS Number:101080-38-0
- Molecular formula:C8H7BrN2O2
- Molecular Weight:243.06
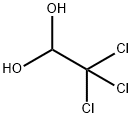
302-17-0
452 suppliers
$30.00/1mg
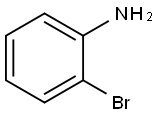
615-36-1
408 suppliers
$10.00/5g

101080-38-0
18 suppliers
$648.80/1G
Yield:101080-38-0 86%
Reaction Conditions:
with hydrogenchloride;hydroxylamine hydrochloride;sodium sulfate in water at 35 - 85; for 3 h;
Steps:
4.2.14. 7-Bromo-1H-indole-2,3-dione (19)
Chloral hydrate (5.01 g, 30.2 mmol) and Na2SO4 (35.0 g, 246 mmol) were dissolved in H2O (70 mL) in a 300 mL beaker and heated to 35 °C. 2-Bromoaniline (4.75 g, 3.12 mL, 27.6 mmol) in H2O (20 mL) and concentrated HCl (3 mL) were warmed and added to the reaction mixture. Hydroxylamine HCl (6.10 g, 87.8 mmol) in H2O (27.5 mL) was warmed and added before the mixture was heated at 85 °C for 3 h. The mixture was cooled, filtered and washed with H2O (100 mL) to yield the intermediate (5.75 g, 86%) as a fluffy light brown powder. Concentrated H2SO4 (100 mL) was heated in a 1 L beaker to 60 °C. The intermediate was added in portions over 15 min so the temperature did not exceed 65 °C. The reaction mixture was then heated at 80 °C for 15 min before being poured into ice (500 mL). The precipitate was washed with H2O (100 mL) to yield 19 as a fine dark red powder (2.94 g, 47%).
References:
Matesic, Lidia;Locke, Julie M.;Vine, Kara L.;Ranson, Marie;Bremner, John B.;Skropeta, Danielle [Tetrahedron,2012,vol. 68,# 34,p. 6810 - 6819] Location in patent:experimental part

302-17-0
452 suppliers
$30.00/1mg
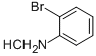
94718-79-3
53 suppliers
$45.00/250mg

101080-38-0
18 suppliers
$648.80/1G
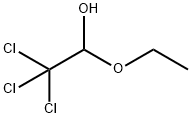
515-83-3
41 suppliers
$67.15/1gm:

615-36-1
408 suppliers
$10.00/5g

101080-38-0
18 suppliers
$648.80/1G

349637-17-8
1 suppliers
inquiry

101080-38-0
18 suppliers
$648.80/1G