2H-1-Benzopyran-2-one, 6-fluoro-3-nitro- synthesis
- Product Name:2H-1-Benzopyran-2-one, 6-fluoro-3-nitro-
- CAS Number:907212-86-6
- Molecular formula:C9H4FNO4
- Molecular Weight:209.13
Yield:907212-86-6 55%
Reaction Conditions:
with diethyl amine hydrochloride in toluene;Heating / reflux;
Steps:
105.1
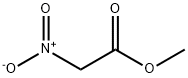
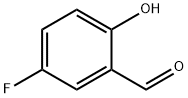
[0359] 5-Fluoro-2-hydroxybenzaldehyde (7.14 g, 50.96 mmol), diethyl amine hydrochloride (6.7Og, 61.15 mmol) and methyl nitroacetate (5.62 mL, 61.15 mmol) were added together in toluene (90 mL) and stirred together at reflux under a N2 atmosphere. A Dean-Stark apparatus was fitted to the system to remove the H2O generated by the reaction. The reaction was allowed to reflux overnight, after which the mixture was cooled and diluted with H2O. The organic layer was separated, washed with brine, dried over anhydrous Na2SO4 and concentrated. Purification by flash chromatography on silica gel afforded the product as an orange solid (5.90 g, 55%)
References:
WO2006/89053,2006,A2 Location in patent:Page/Page column 76