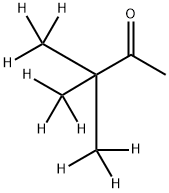
[2H9]-Pinacolone synthesis
- Product Name:[2H9]-Pinacolone
- CAS Number:42983-09-5
- Molecular formula:C6H12O
- Molecular Weight:100.16
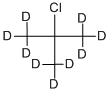
918-20-7
36 suppliers
$140.00/100mg

75-36-5
560 suppliers
$17.92/100G
![[2H9]-Pinacolone](/CAS/20211123/GIF/42983-09-5.gif)
42983-09-5
4 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: tert-butyl chloride-d9with magnesium;iodine in diethyl ether;Reflux;
Stage #2: acetyl chloride in diethyl ether at -5 - 20;
Steps:
3.1
[137] Step 1. 4A4-dr3,3-Bis(methyl-d0butan-2-one (15): A four-neck round bottom flask equipped with a mechanical stirrer, thermowell, nitrogen inlet and dropping funnel was charged with diethyl ether (10 mL), magnesium powder (2.36 g, 98 mmol) and a small crystal of iodine. Approximately 10 mL of a solution of t-butyl chloride-d9 (10 g, 98 mmol; Cambridge Isotopes, 98 atom% D) in diethyl ether (100 mL) was added. The solution was warmed slightly as needed to help initiate the reaction. The t-butyl chloride-d9 solution was then added at such a rate as to maintain reflux. After the addition the reaction was maintained at reflux for a further 2 hr until most of the magnesium had been consumed. The thick grey suspension was cooled to room temperature. The cooled mixture was slowly cannulated into cold (-5-0 0C) neat acetyl chloride (21 mL, 295 mmol) at such a rate as to maintain the temperature at less than 5 0C. The greenish- yellow suspension was allowed to warm to room temperature and was stirred overnight. The mixture was cooled to 0 0C and 4M HCl (60 mL) was added slowly to quench the reaction. The mixture was saturated with solid NaCl. Three layers were observed and separated. The lower (mostly brine) and middle (mostly AcOH) layers were washed with diethyl ether. The combined organic solutions were stirred over solid NaHCO3 with small amounts (1 mL) of water periodically added until an NMR of an aliquot showed most of the acetic acid had been consumed. The solution was dried (Na2SO4), filtered, and concentrated using a cool (less than 30 0C) water bath to afford 3.8 g (36%) of 15. The material was carried forward to the next step.
References:
WO2011/11712,2011,A1 Location in patent:Page/Page column 35-36
75160-23-5
0 suppliers
inquiry
![[2H9]-Pinacolone](/CAS/20211123/GIF/42983-09-5.gif)
42983-09-5
4 suppliers
inquiry