
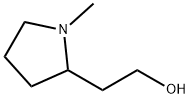
(2R)-1-Methyl-2-Pyrrolidineethanol synthesis
- Product Name:(2R)-1-Methyl-2-Pyrrolidineethanol
- CAS Number:60307-26-8
- Molecular formula:C7H15NO
- Molecular Weight:129.2

101555-60-6
123 suppliers
$15.00/100mg

60307-26-8
27 suppliers
$137.00/50mg
Yield:60307-26-8 82%
Reaction Conditions:
Stage #1: 2-(R)-carboxymethyl-pyrrolidine-1-carboxylic acid tert-butyl esterwith lithium aluminium tetrahydride in 1,4-dioxane at 0 - 70;Reflux;
Stage #2: with water in 1,4-dioxane at 0;
Steps:
1.6
(R)-2-(1-Methylpyrrolidin-2-yl)ethanol: At about 0° C., a solution of (R)-2-carboxymethyl-pyrrolidine-1-carboxylic acid tert-butyl ester (1.30 g, 5.68 mmol) in dry 1,4-dioxane (10 mL) was added dropwise to a stirred suspension of lithium aluminum hydride (1.08 g, 28.46 mmol) in dry 1,4-dioxane (10 mL). With slow temperature ramping, the mixture was first heated at about 50° C. for about 1 hour, then heated at about 70° C. for about 1 hour, and finally heated at reflux for about 5 hours. After cooling the mixture to about 0° C., cold water was added dropwise until a white granular precipitate was obtained. The precipitate was collected by filtration, washed with ethyl acetate, and the washes were combined with the filtrate. The combined filtrate was concentrated in vacuo to yield the title compound as a brown oil which was used in the next step without further purification (0.600 g, 82%). 1H NMR (400 MHz, DMSO-d6) δ 1.46-1.56 (m, 1H), 1.72-1.84 (m, 3H), 1.87-2.05 (m, 2H), 2.12-2.20 (m, 1H), 2.36 (s, 3H), 2.56-2.63 (m, 1H), 3.03-3.09 (m, 1H), 3.65-3.72 (m, 1H), 3.96-4.03 (m, 1H); IR (KBr) υ 3379, 2955, 2789, 1655, 1458 cm-1; MS 130 (M+1).
References:
US2009/203763,2009,A1 Location in patent:Page/Page column 34
67004-64-2
205 suppliers
$12.00/1g

60307-26-8
27 suppliers
$137.00/50mg
107870-33-7
1 suppliers
inquiry

60307-26-8
27 suppliers
$137.00/50mg