
(2R)-2-benzhydryloxirane synthesis
- Product Name:(2R)-2-benzhydryloxirane
- CAS Number:862647-09-4
- Molecular formula:C15H14O
- Molecular Weight:210.27
Yield:97 % ee
Reaction Conditions:
Stage #1: (1R,2R)-(-)-N,N'-bis(3,5-di-tert-butylsalicydene)-1,2-cyclohexanediaminocobalt(II);acetic acid in toluene at 20; for 1 h;
Stage #2: 1,1-diphenyl-2,3-epoxy-propan in water at 0 - 20; for 72 h;Hydrolytic kinetic resolution;
Steps:
Resolution of Racemic 2-benzhydryl-oxirane (23) by HKR Reaction A mixture of (R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2- cyclohexane diaminocobalt (II) (0.22 g, 0.37 mmol, 0.8%), toluene (5 ml), and acetic acid (0.044 g, 0.74 mmol) was stirred for Ih at room temperature. The solvent was removed in vacuo and the residue was dried. 2-Benzhydryl-oxirane (9.6 g, 45.7 mmol) was added in one portion and stirred, the mixture was then cooled by means of an ice-bath. H20 (0.58 g, 32 mmol) was slowly added over a 30-min period. After water addition, the ice bath was removed and the reaction mixture was stirred at room temperature for 72h. Compounds were separated via flash chromatography on slica gel column to give (2R) -2-benzhydryl-oxirane (23a) 4.5 g ([α]D=(+)9.58, c=1, MeOH) and (25)-3 ,3-diphenyl-propane-1 ,2-diol 24 3.53 g ([ex]D=( + )48, c=1, MeOH, ee=97%). The proton and carbon NMR data of (2R)- 2-benzhydryl-oxirane was identical to the racemate 2-benzhydryl-oxirane. (at)HNMR (CDCl3,400MHz) 2.54 (m, IH, H-1) 2.87 (m, 1H, H-1) 3.54 (m, IH, H-2) 3.86 (d, 1H, Ph2CH), 7.2-7.4 (m, 10H, aromatic-H). 13CNMR (CDCl3, 100MHz) 46.80, 53.58, 55.17, 127.06, 127.14, 128.70, 128.81, 141.28. For (2S)-3,3-diphenyl-propane-1,2-diol: (at)HNMR (CDCl3,400MHz) 2.39 (bs, 2H, OH) 3.44 (m, 1H,H-1) 3.60 (m, 1H, H-1), 4.02 (D, J=10Hz, IH, Ph2CH), 4.44 (m, 1H, H-2), 7.16- 7.22 (m, 10H, aromatic-H). 13CNMR (CDCl3,100MHz) 55.08,64.94, 74.26, 127.08,127.23, 128.35,128.84, 129.03, 129.17, 141.23, 141.62
References:
WO2005/105075,2005,A1 Location in patent:Page/Page column 40; Sheet 6

3542-14-1
0 suppliers
inquiry

862647-09-4
15 suppliers
inquiry
101-81-5
401 suppliers
$7.00/25g

862647-09-4
15 suppliers
inquiry
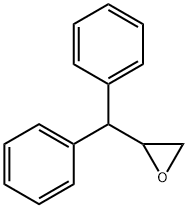
15701-83-4
1 suppliers
inquiry

862647-09-4
15 suppliers
inquiry

4646-55-3
3 suppliers
inquiry

862647-09-4
15 suppliers
inquiry
