
(2R)-2-(Methoxymethyl)morpholine synthesis
- Product Name:(2R)-2-(Methoxymethyl)morpholine
- CAS Number:157791-21-4
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
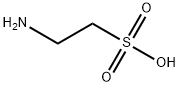
107-35-7
974 suppliers
$5.00/5g

64491-70-9
99 suppliers
$75.00/500mg

157791-21-4
36 suppliers
$119.00/50mg
Yield: 10%
Reaction Conditions:
with sodium hydroxide in methanol at 50; for 21.25 h;
Steps:
15-1-A
Synthesis 15-1 -A; (f?)-2-(Methoxymethyl)morpholine; A solution of (R)-(~) glycidylmethylether (0.800 g, 10.66 mmol) in methanol (11 mL) was added dropwise to a solution of 2-aminoethanesulfonic acid (6.440 g, 53.3 mmol) in 40% aqueous sodium hydroxide (11 mL) at 5O0C. After stirring for 75 minutes, further 40% aqueous sodium hydroxide (19 mL) was added and the reaction mixture was stirred for 20 hours at 5O0C. The solution was cooled to room temperature and diluted with water (76 mL). The aqueous phase was extracted with ethyl acetate (3 x 75 mL). The combined organic phases were dried (Na2SO4). The solvent was removed in vacuo and
References:
CANCER RESEARCH TECHNOLOGY LIMITED WO2008/75007, 2008, A1 Location in patent:Page/Page column 106-107