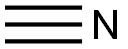(2RS,3S)-AHPA synthesis
- Product Name:(2RS,3S)-AHPA
- CAS Number:209173-80-8
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
![Carbamic acid, N-[(1S)-2-cyano-2-hydroxy-1-(phenylmethyl)ethyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/186393-18-0.gif)
186393-18-0
0 suppliers
inquiry

209173-80-8
9 suppliers
inquiry
Yield:209173-80-8 88%
Reaction Conditions:
with hydrogenchloride;water in 1,4-dioxane; for 18 h;Reflux;
Steps:
8.3
(3) N-(tert-l3utoxycarbonyl)-L-phenylalaninal (17 g, 67 mmol) was dissolved in methanol (100 mE), and the solution was cooled to 5° C. Sodium bisulfite (7.0 g, 67 mmol) was dissolved in water (150 mE) and cooled to 5° C. The solution was added to an aldehyde solution, and the mixture wasstirred at 5° C. for 18 hours. Sodium cyanide (4.0 g, 81 mmol) was dissolved in water (100 mE), and added with ethyl acetate (300 mE) to the above reaction mixture. The reaction solution was stirred at room temperature for 5 hours. The organic layer was separated, dehydrated with anhydrous magnesium sulfate, and concentrated in vacuo to yield cyanohydrin ascolorless oil. The cyanohydrin was dissolved in dioxane (250 mE) and concentrated hydrochloric acid (250 mE), followed by the addition of anisole (10 mE). This solution was gently refluxed for 18 hours. The reaction solution was cooled to room temperature and then concentrated in vacuo to yieldbrown semi-solid substance. The brown semi-solid substance was dissolved in water (100 mE) and washed with diethyl ether (3x50 mE). The aqueous layer was then applied tocolumn of Dowex 50x8 (100 to 200 mesh, H+ type; 25x1.8 cm), washed with water until the pH reached 5.5, and eluted with 2M ammonia water (about 1.5 E). The eluted ammonia water was concentrated in vacuo to yield (35)-3-amino-2- hydroxy-4-phenylbutyric acid (12 g, 88%) as a white solid.
References:
US9371359,2016,B2 Location in patent:Page/Page column 35
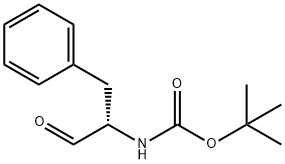
72155-45-4
170 suppliers
$22.00/250mg

209173-80-8
9 suppliers
inquiry
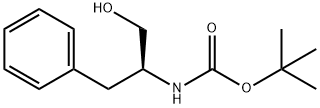
66605-57-0
325 suppliers
$6.00/1g

209173-80-8
9 suppliers
inquiry

87694-53-9
61 suppliers
$33.00/1g

209173-80-8
9 suppliers
inquiry