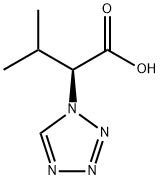
(2S)-3-Methyl-2-(1H-tetrazol-1-yl)butanoic acid synthesis
- Product Name:(2S)-3-Methyl-2-(1H-tetrazol-1-yl)butanoic acid
- CAS Number:1218381-05-5
- Molecular formula:C6H10N4O2
- Molecular Weight:170.1692
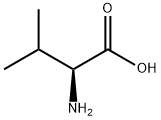
72-18-4
812 suppliers
$5.00/25g
122-51-0
485 suppliers
$10.00/5ml

1218381-05-5
7 suppliers
$315.00/500mg
Yield:1218381-05-5 70%
Reaction Conditions:
with sodium azide;acetic acid at 80; for 6 h;
Steps:
General Experimental Procedure for the Synthesis of 1-substituted-1H-1,2,3,4-tetrazoles from L-α-amino Acids
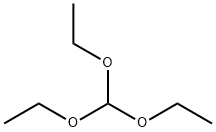
General procedure: L--amino acid (0.01 mole) and sodium azide (0.65 g,0.01 mole) were added to a solution of TEOF (4.4 g, 5 mL,0.03 mole) and glacial acetic acid (20 mL) while stirring andthe mixture was heated at 80 °C for 1-7 h depending substrates(Table 1). The reaction mixture was then cooled andevaporated in a vacuum. For entries 1-3, the residue wasdissolved in acetone (50 mL), filtered and the filtrate wasevaporated in a vacuum. Distilled water was added (20 mL)and the pH of the solution adjusted to 9-10 with NaOH solution(5 M). The solution was refluxed with activated carbon(1.5 g) for 30 min, filtered, cooled and the pH adjusted to 2with HCl solution (2 M). Due to the low pKa of 1Htetrazoles(ca. 3-5) and their high crystalline nature [24],products were precipitated, separated and dried in vacuum at60 °C.For entries 4 and 5, after removal of HOAc, the residuewas dissolved in acetone (50 mL), and filtered, the filtrateconcentrated to 5 mL, CH2Cl2 (15 mL) added and allowedfor slow precipitation. The precipitate was filtered off to givethe white crystalline products.For entries 6-8 (Table 1), after removal of HOAc, theresidue was dissolved in distilled water, the pH adjusted to 9adding a solution of NaOH (5 M) and refluxed with activatedcarbon (1.5 g) for 30 min. After cooling, the solution wasfiltered, the pH of filtrate adjusted to 7 adding a solution ofHCl (1 M) and then evaporated in a vacuum. The crudeproduct was dissolved in dry ethanol (20 mL), cooled in afridge for 1h and filtered; the filtrate was concentrated to 10mL and acetone (70 mL) added to filtrate and allowed toslowly precipitate in a fridge. The precipitate was filtered offto give tetrazoles (55-80 %). All products were characterizedby NMR, FT-IR, elemental analysis (CHN), and meltingpoints.
References:
Habibi, Davood;Rahmani, Payam;Ahmadi, Fatemeh;Bokharaei, Hanieh;Kaboudvand, Zahra [Letters in Organic Chemistry,2014,vol. 11,# 2,p. 145 - 151]