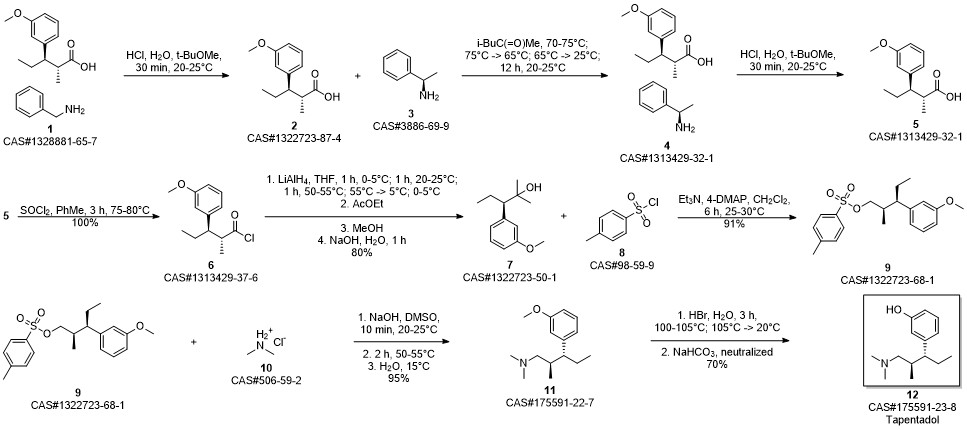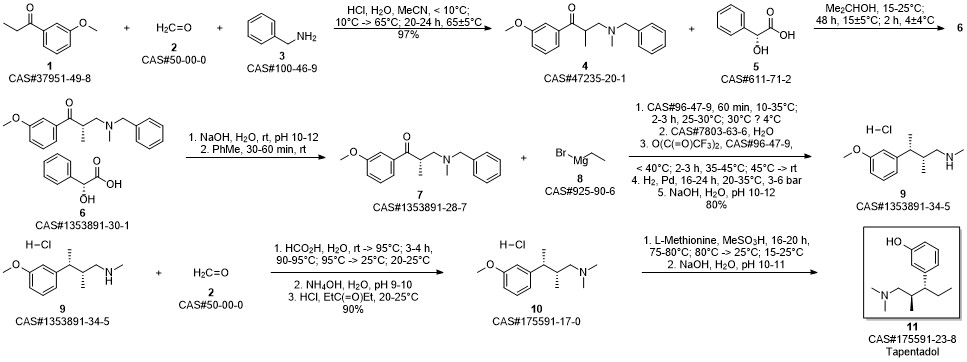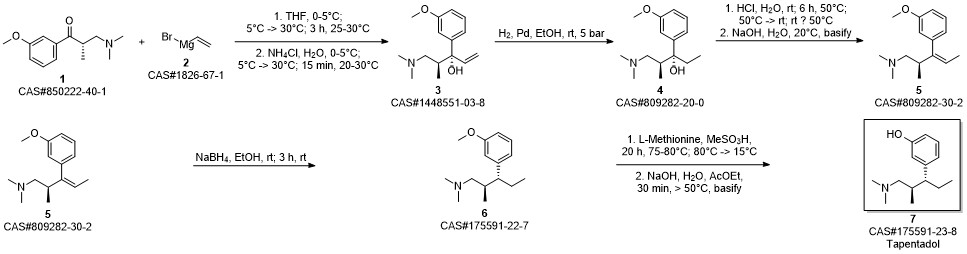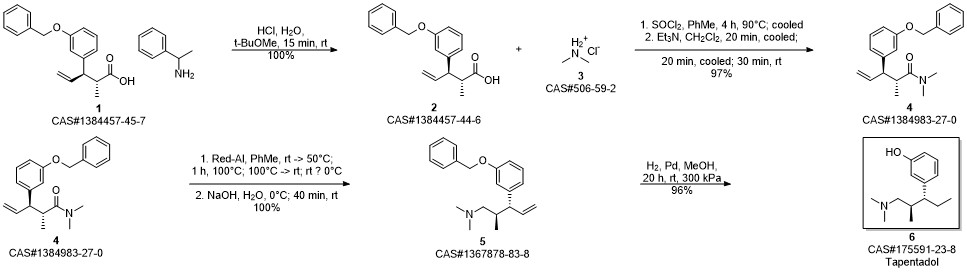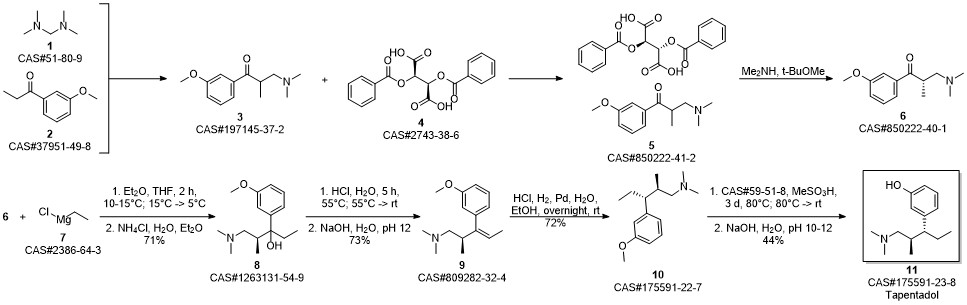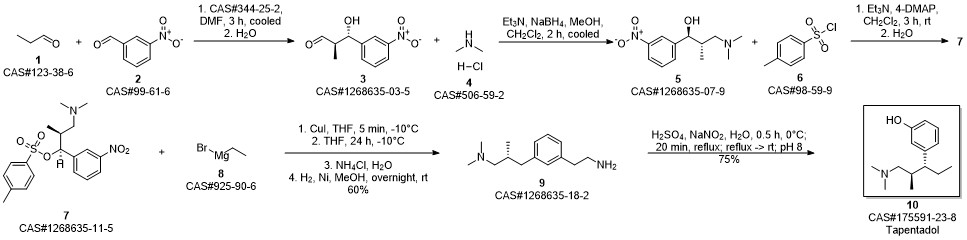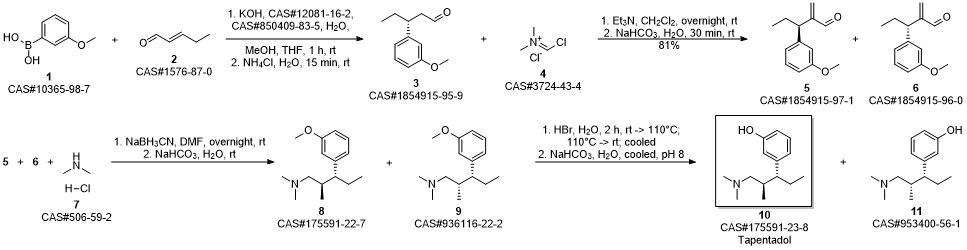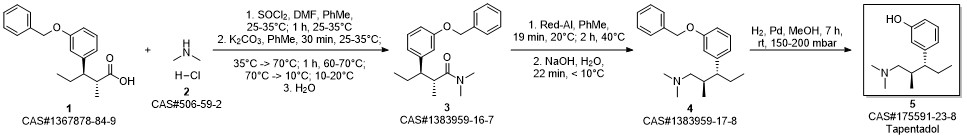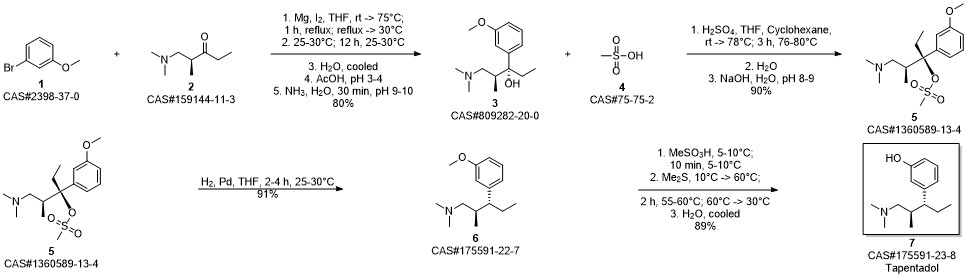
3-(1-dimethylamino-2-methyl-pentan-3-yl)phenol synthesis
- Product Name:3-(1-dimethylamino-2-methyl-pentan-3-yl)phenol
- CAS Number:175591-23-8
- Molecular formula:C14H23NO
- Molecular Weight:221.34
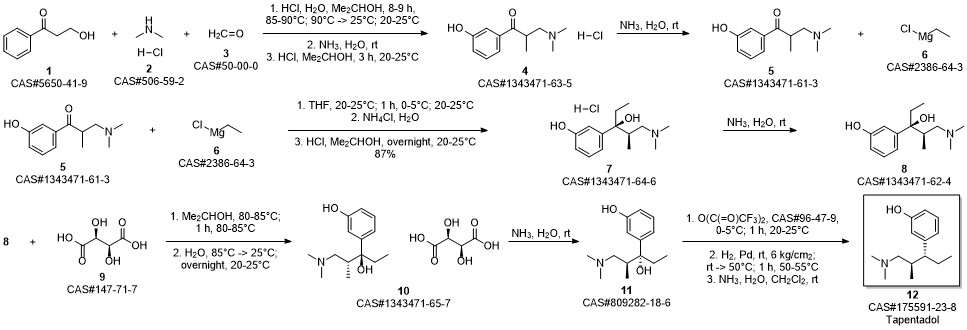
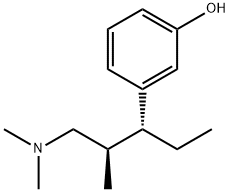
Khunt, Mayur Devjibhai; Bondge, Sandipan Prabhurao; Patil, Nilesh Sudhir; Pagire, Haushabhau Shivaji; Pradhan, Nitin Sharadchandra. Preparation of highly pure tapentadol or a pharmaceutically acceptable salt. Assignee Actavis Group PTC EHF, Iceland. WO 2011128784. (2011).

175591-22-7
55 suppliers
$504.97/5MG

175591-23-8
0 suppliers
inquiry
Yield:175591-23-8 98%
Reaction Conditions:
with hydrogen bromide in water at 100 - 110; for 3 h;
Steps:
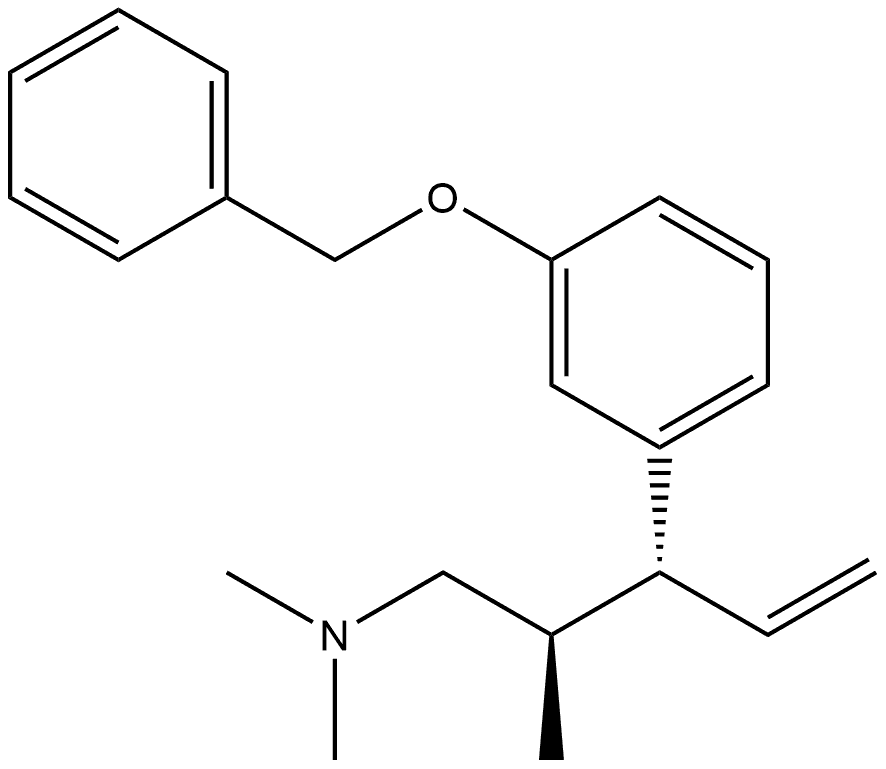
15-17 Example 15 : 3-(l-(dimethylamino)-2-methylpentan-3-yl) phenol (or Tapentadol):
A mixture of 3-(3-methoxyphenyl)-/V,/V-2-trimethylpentan- 1-amine, from Examples 12, 13 or 14 (1 g, 4.2 mmol) and aqueous hydrobromic acid (HBr) (46 %, 20 ml) was heated under stirring at 100-110 °C for 3 h. The reaction mixture was then cooled to room temperature. The reaction mixture was neutralized with sodium bicarbonate. Resulting product was extracted with ethyl acetate. The organic layer was washed with water and dried over anhydrous sodium sulphate and concentrated to give 3-(l-(dimethylamino)-2-methylpentan- 3-yl) phenol (I) (921 mg, 98 %) as a brownish oil. In HPLC analysis anti and syn diastereomers were found to be 96.4:3.4 ratio i.e. 96.4% purity of the desired anti diastereoisomer was obtained, as measured using HPLC.NMR data for anti diastereomer, 1H NMR (500 MHz, CDCI3) d 7.12 (t, J = 7.8 Hz, 1H), 6.68 - 6.61 (m, 2H), 6.58 (s, 1H), 2.33 - 2.27 (m, 1H), 2.17 (s, 6H), 2.15 - 2.10 (m, 1H), 2.09 - 2.00 (m, 1H), 1.90 - 1.83 (m, 1H), 1.78 - 1.68 (m, 1H), 1.60 - 1.49 (m, 1H), 0.96 (d, J =6.7 Hz, 3H), 0.70 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCI3) d 156.5, 146.1, 129.2, 120.3, 115.8, 113.3, 64.8, 51.5, 45.7, 36.6, 23.9, 16.2, 12.4; IR (neat) nmax 3391, 2958, 2871, 1695, 1464, 1266, 1029, 775; HRMS (ESI) calcd for C 14H24NO [M+H]+: 222.1858; found: 222.1865. NMR data for syn diastereomer, 1H NMR (400 MHz, CDCI3) d 7.10 (t, J = 7.8 Hz, 1H), 6.65 (dd, J = 8.0, 2.1 Hz, 2H), 6.59 - 6.55 (m, 1H), 2.46 - 2.37 (m, 1H), 2.29 - 2.25 (m, 1H), 2.24 (s, 6H), 2.02 - 1.94 (m, 1H), 1.94 - 1.82 (m, 1H), 1.78 - 1.58 (m, 2H), 0.80 - 0.71 (m, 6H); 13C NMR (101 MHz, CDCI3) d 156.2, 144.6, 128.9, 121.4, 116.0, 113.4, 77.5, 77.2, 76.8, 65.4, 50.7, 45.9, 35.8, 26.8, 15.6, 12.7; IR (neat) nmax 3310, 2951, 2865, 1595, 1465, 1263, 1028, 779; HRMS (ESI) calcd for C 14H24NO [M+H]+: 222.1858; found: 222.1878.
References:
COUNCIL OF SCIENTIFIC AND INDUSTRIAL RESEARCH;SRIVARI, Chandrasekhar;PRATHAMA, Satyendra Mainkar;RAMAGONOLLA, Kranthi Kumar;GENJI, Sukumar WO2020/194326, 2020, A1 Location in patent:Page/Page column 6; 15-16
1367878-83-8
0 suppliers
inquiry

175591-23-8
0 suppliers
inquiry
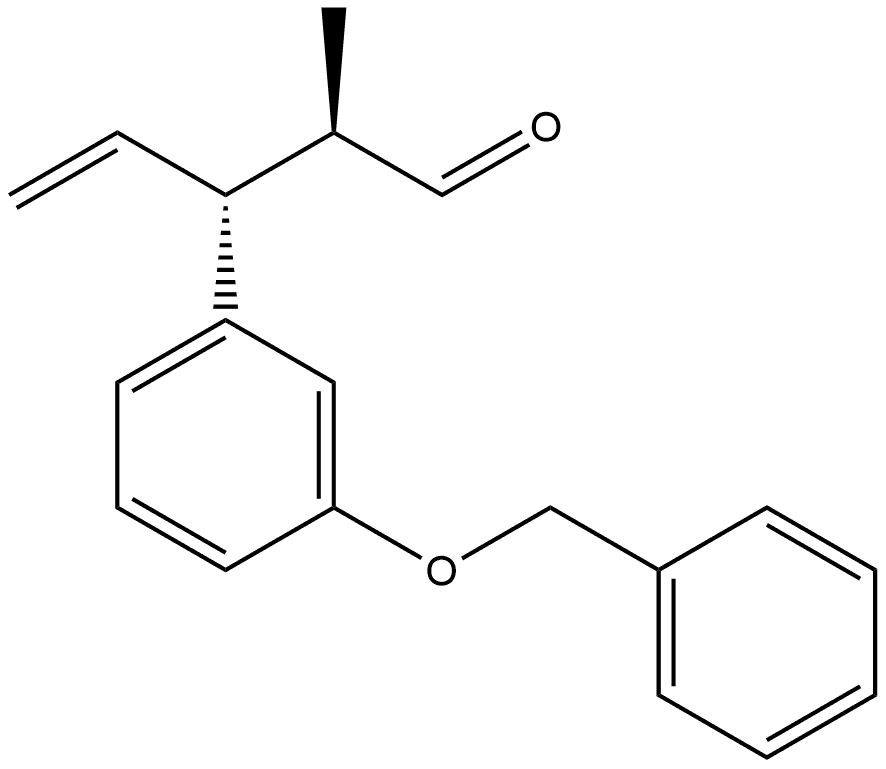
1443156-34-0
0 suppliers
inquiry

124-40-3
531 suppliers
$18.00/100ml

175591-23-8
0 suppliers
inquiry
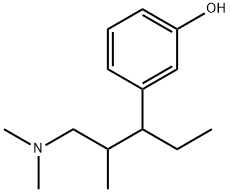
515114-52-0
1 suppliers
inquiry

175591-23-8
0 suppliers
inquiry