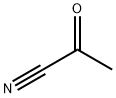
3-(1-METHYL-CYCLOPROPYL)-3-OXO-PROPIONITRILE synthesis
- Product Name:3-(1-METHYL-CYCLOPROPYL)-3-OXO-PROPIONITRILE
- CAS Number:88485-78-3
- Molecular formula:C7H9NO
- Molecular Weight:123.15
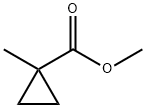
6206-25-3
69 suppliers
$45.00/100mg
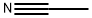
75-05-8
966 suppliers
$10.00/10g

88485-78-3
30 suppliers
inquiry
Yield:88485-78-3 99%
Reaction Conditions:
Stage #1: acetonitrilewith n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2: methyl 1-methylcyclopropane-1-carboxylate in tetrahydrofuran;hexane at -78 - -10; for 3 h;
Steps:
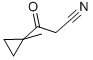
3-(1-Methylcyclopropyl)-3-oxopropanenitrile (59b)
To a cooled stirred -78 °C solution of nBuLi (2.5 M solution in hexanes, 6.80 mL, 17.0 mmol) inTHF (30 mL) was added acetonitrile (0.89 mL, 17 mmol). After 1 h, methyl 1-methylcyclopropane-1-carboxylate (59a, 1.00 mL, 8.50 mmol) was added and the resulting mixture was slowly warmedto -10 °C over 3 h. The reaction was quenched with 2 N HCl (15 mL), and then diluted with diethylether (100 mL). The separated organic layer was dried over sodium sulfate, filtered, andconcentrated under reduced pressure to afford 3-(1-methylcyclopropyl)-3-oxopropanenitrile (59b) as a pale yellow oil (1.09 g, >99%) that was suitable for use without further purification: 1H NMR(300 MHz, CDCl3) 3.59 (s, 2H), 1.41 (s, 3H), 1.39-1.31 (m, 2H), 0.93-0.85 (m, 2H).
References:
Barnes, Keith D.;Buckle, Ronald N.;Chen, Xinchao;Herr, R. Jason;Johnson, Graham;Lin, Juinn H.;Mayhew, Nicholas J.;Mobley, William C.;Nguyen, Phuong;Paquette, William D.;Rynearson, Kevin D.;Sakwa, Samuel A.;Tanzi, Rudolph E.;Wagner, Steven L.;Yang, Jinhai [Bioorganic and Medicinal Chemistry,2020,vol. 28,# 22,art. no. 115734] Location in patent:supporting information

6206-25-3
69 suppliers
$45.00/100mg

88485-78-3
30 suppliers
inquiry
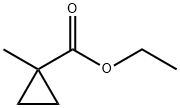
71441-76-4
93 suppliers
$17.00/250mg
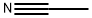
75-05-8
966 suppliers
$10.00/10g

88485-78-3
30 suppliers
inquiry