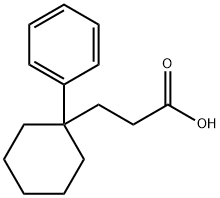
3-(1-PHENYLCYCLOHEXYL)PROPANOIC ACID synthesis
- Product Name:3-(1-PHENYLCYCLOHEXYL)PROPANOIC ACID
- CAS Number:7598-04-1
- Molecular formula:C15H20O2
- Molecular Weight:232.32
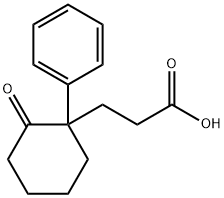
2819-68-3
11 suppliers
inquiry

7598-04-1
7 suppliers
$356.00/250mg
Yield:7598-04-1 93%
Reaction Conditions:
Stage #1: 3-(2-oxo-1-phenyl-cyclohexyl)-propionic acidwith hydrazine hydrate;potassium hydroxide in ethylene glycol at 130; for 3.5 h;Reflux;
Stage #2: with hydrogenchloride in water;ethylene glycol at 20;
Steps:
1.1C
Preparation 1C: 3-(l-phenylcyclohexyl)propanoic acid (1C)(1C)[0130] A mixture of 3-(2-oxo-l-phenylcyclohexyl)propanoic acid (1 g, 4.06 mmol), potassium hydroxide (0.770 g, 13.72 mmol), hydrazine monohydrate (0.348 mL, 6.09 mmol) and ethylene glycol (5 mL) was heated to 130°C for 1.5h. The condenser was removed and the mixture was heated to 200°C for 30min. The reaction mixture was then heated under reflux with a reflux condenser for additional 1.5h. The reaction mixture was cooled to room temperature and diluted with IN HCl to a total reaction volume of 25 mL. The precipitated product was collected by filtration, washed several times with water, and dried in vacuo to obtain 3-(l-phenylcyclohexyl)propanoic acid as a white solid (1C, 923 mg, 93% yield). HPLC: tR = 3.7 min, Chromolith SpeedRod 4.6 x 50 mm, 4 min gradient, Detection Wave length 220 nm, Starting solvent: 10% aq. MeOH- 0.2% H3P04; Final solvent: 90% aq. MeOH-0.2% H3P04. LC/MS: m/e 215.19 (M+H-H20)+.Example 1 :
References:
WO2012/12477,2012,A1 Location in patent:Page/Page column 51-52
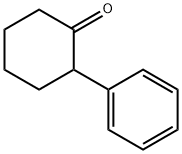
1444-65-1
136 suppliers
$12.00/250mg

7598-04-1
7 suppliers
$356.00/250mg
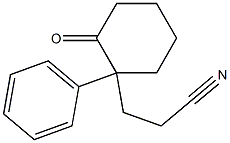
32231-10-0
0 suppliers
inquiry

7598-04-1
7 suppliers
$356.00/250mg