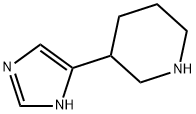
3-(1H-IMIDAZOL-4-YL)-PIPERIDINE synthesis
- Product Name:3-(1H-IMIDAZOL-4-YL)-PIPERIDINE
- CAS Number:784080-46-2
- Molecular formula:C8H13N3
- Molecular Weight:151.21
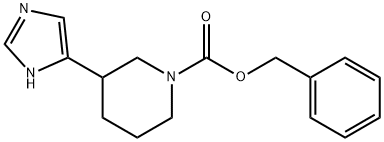
1352654-87-5
0 suppliers
inquiry

784080-46-2
10 suppliers
inquiry
Yield:784080-46-2 31%
Reaction Conditions:
Stage #1: benzyl 3-(1H-imidazol-5-yl)piperidine-1-carboxylatewith hydrogenchloride in 1,4-dioxane;water at 80; for 16 h;
Stage #2: with sodium hydrogencarbonate in water;
Steps:
21.2
Step 2. 3-(1H-Imidazol-5-yl)piperidine; A 100-mL round-bottomed flask was charged with a solution of benzyl 3-(1H-imidazol-5-yl)piperidine-1-carboxylate (3 g, 10.53 mmol, 1.00 equiv) in 1,4-dioxane (30 mL) and conc. HCl (18 mL). The resulting mixture was heated to 80° C. in an oil bath for 16 hours. The reaction progress was monitored by TLC (DCM: MeOH=10:1). Upon completion, the resulting mixture was concentrated on a rotary evaporator. The resulting solution was diluted with water (60 mL) and treated with aqueous NaHCO3. The resulting mixture was then extracted with dichloromethane (4×40 mL). The combined organic layers were dried over anhydrous sodium sulfate, filtered off and concentrated on a rotary evaporator affording 3-(1H-imidazol-5-yl)piperidine as brown oil (0.5 g, 31%).
References:
US8080566,2011,B1 Location in patent:Page/Page column 51

201478-72-0
80 suppliers
$45.00/25mg

784080-46-2
10 suppliers
inquiry
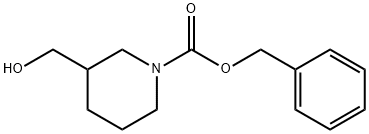
39945-51-2
126 suppliers
$19.00/1g

784080-46-2
10 suppliers
inquiry
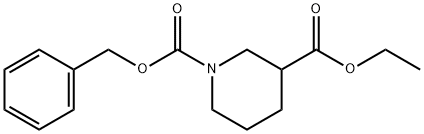
310454-53-6
83 suppliers
$15.00/1g

784080-46-2
10 suppliers
inquiry

71962-74-8
48 suppliers
inquiry

784080-46-2
10 suppliers
inquiry