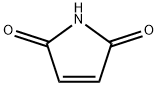
3-(1H-indol-3-yl)pyrrolidine-2,5-dione synthesis
- Product Name:3-(1H-indol-3-yl)pyrrolidine-2,5-dione
- CAS Number:10185-29-2
- Molecular formula:C12H10N2O2
- Molecular Weight:214.22
Yield:10185-29-2 89%
Reaction Conditions:
with acetic acid for 48 h;Inert atmosphere;Reflux;
Steps:
3-(1H-Indol-3-yI)pyrrolidine-2,5-dione (64)
Modified from references Andrianjara et al. (2002), Da Silva et al. (2009) and Hamilton et al. (1995), indole (6a) (10.0 mmol) and maleimide (63) (30.0 mmol) were dissolved in acetic acid (50.0 mL) under nitrogen atmosphere and refluxed for 48 h (TLC monitoring). The reaction mixture was concentrated on a rotary evaporator and dissolved again in ethyl acetate (50.0 mL) and sat. NaHCO3 solution (50.0mL). The mixture was extracted with ethyl acetate (2 x 50.0 mL). The organic phases were combined, dried over sodium sulfate and concentrated on a rotary evaporator. The oily residue was purified by column chromatography on silica gel (mobile phase: dichloromethane / ethyl acetate 2:1). 1.92 g (8.97 mmol, 89 %) yellow crystals; NMR (300 MHz, DMSO-d6): δ 1 1.28 (s, 1H), 1 1.03 (s, 1H), 7.42 (d, J - 7.9 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.33 (d, J = 2.4 Hz, 1H), 7.10 (m, 1H), 7.00 (m, 1H), 4.33 (dd, J= 9.5, 5.3 Hz, 1H), 3.18 (dd, J= 18.0, 9.5 Hz, 1H), 2.75 (m
References:
WO2016/20369,2016,A1 Location in patent:Page/Page column 121
125314-92-3
0 suppliers
inquiry

10185-29-2
3 suppliers
inquiry
![2,5-Pyrrolidinedione, 3-[5-(phenylmethoxy)-1H-indol-3-yl]-](/CAS/20210305/GIF/681163-03-1.gif)
681163-03-1
0 suppliers
inquiry

10185-29-2
3 suppliers
inquiry
120-72-9
622 suppliers
$6.00/25g

10185-29-2
3 suppliers
inquiry
1215-59-4
330 suppliers
$10.00/1g

10185-29-2
3 suppliers
inquiry