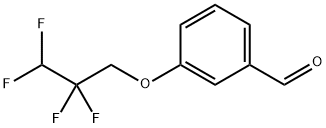
3-(2,2,3,3-tetrafluoropropoxy)benzaldehyde synthesis
- Product Name:3-(2,2,3,3-tetrafluoropropoxy)benzaldehyde
- CAS Number:133487-66-8
- Molecular formula:C10H8F4O2
- Molecular Weight:236.16
Yield:133487-66-8 54%
Reaction Conditions:
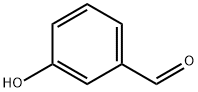
Stage #1: meta-hydroxybenzaldehydewith potassium carbonate in N,N-dimethyl-formamide; for 0.0833333 h;Cooling with ice;
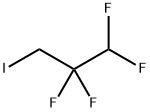
Stage #2: 1-iodo-1,1,3-trihydroperfluoropropane in N,N-dimethyl-formamide; for 2 h;Heating;
Steps:
3-(2,2,3,3-tetrafluoropropoxy)benzaldehyde 13.
K2CO3 (6.79 g, 49.13 mmol, 3.0 equiv) was added to a solution of 3- hydroxybenzaldehyde (1.0 g, 8.19 mmol, 1.0 eq) in DMF (70 mL), with an ice bath cooling. After 5 min, the 1,1,2,2-tetrafluoro-3- iodopropane (1.4 mL, 12.28 mmol, 1.5 equiv) was added in the mixture. The solution was then stirred for 2 h at 70 °C. After concentrating the mixture in vacuo, the crude was dissolved with EtOAc, then the organic layer was washed with brine, dried over MgSO4 and concentrated in vacuo. The crude was purified by chromatography on silica gel column (CHX/EtOAc, gradient 100:0 to 95:5) and concentrated under reduced pressure to afford the compound 13 as a colorless oil (815 mg, 54% isolated yield). 1 H NMR (CDCl3, 400 MHz, 295 K) δ 9.98 (s, 1H), 7.56 (td, 1H, J = 7.8, 1.2 Hz), 7.50 (t, 1H, J = 7.8 Hz), 7.41 (dd, 1H, J = 2.7, 1.2 Hz), 7.22 (dd, 1H, J = 7.8, 2.7, 1.2 Hz), 6.07 (tt, 1H, J = 53.1, 4.2 Hz), 4.41 (tt, 2H, J = 12.1, 1.5 Hz); 13C NMR (CDCl3, 101 MHz, 295 K) δ 191.6, 158.0, 138.1, 130.6, 125.3, 122.0, 114.4 (tt, J = 249.9, 27.2 Hz), 112.9, 109.0 (tt, J = 249.9, 34.7 Hz), 65.2 (t, J = 29.8 Hz); 19F NMR (CDCl3, 376 MHz, 295 K) δ 124.8 (tq, 2F, J = 12.1, 4.3 Hz), 139.1 (dt, 2F, J = 53.1, 3.5 Hz); IR (neat, cm-1 ) νmax 2929, 1706, 1104.
References:
Toublet, Fran?ois-Xavier;Lalut, Julien;Hatat, Bérénice;Lecoutey, Cédric;Davis, Audrey;Since, Marc;Corvaisier, Sophie;Freret, Thomas;Sopková-de Oliveira Santos, Jana;Claeysen, Sylvie;Boulouard, Michel;Dallemagne, Patrick;Rochais, Christophe [European Journal of Medicinal Chemistry,2021,vol. 210]