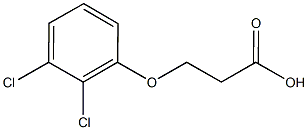
3-(2,3-dichlorophenoxy)propanoic acid synthesis
- Product Name:3-(2,3-dichlorophenoxy)propanoic acid
- CAS Number:7170-58-3
- Molecular formula:C9H8Cl2O3
- Molecular Weight:235.064
Yield:7170-58-3 42%
Reaction Conditions:
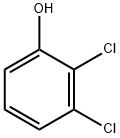
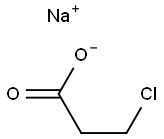
Stage #1: 2,3-dichlorophenolwith sodium hydroxide in water at 100; for 1 h;
Stage #2: β-Propiolactone for 12.08 h;Reflux;
Stage #3: pH=2;Acidic aqueous solution;
Steps:
VIII.3.i
[153] Scheme 3: Synthesis of 2-(2-(6,7-dichloro-4H-chromeno[4,3-d]thiazol-2-yl)hydrazono)- 3-(2-nitrophenyl)propanoic acid (PC202):; [154] Synthesis of 3-(2,3-dichlorophenoxy)propanoic acid (PC164): Procedure: 0.4 g (0.01 mol) of Sodium hydroxide was dissolved in 4 ml of water. To this stirred solution was added 1.63 g (0.01 mol) of 2,3-dichloro phenol was added. Once the solid had dissolved the alkaline solution was kept stirring at 100 C for 1 hour. After that β-propiolactone 0.72 g (0.628 ml, 0.01 mol) was added slowly in drops for 5 minutes. The reaction mixture was further continued heating for 12 h. The reaction mixture was cooled to room temperature and water 10 ml was added. This diluted mixture was acidified by the addition of Con. HC1. The product was extracted twice with diethyl ether (20 ml). The ether layer was then washed with 10 % Sodium bicarbonate. The aqueous layer was acidified to pH= 2. The precipitated solid was filtered and dried to afford the required product 3-(2,3-dichlorophenoxy)propanoic acid.PC164[155] PC 164: White solid, Yield: 1 g (42 %); 1H NMR (CD3OD, 400 MHz) in ppm: δ 2.00 (t, 2H), 4.28 (t, 2H), 6.98-7.00 (m, 1H), 7.06-7.08 (m, 1H), 7.17-7.21 (m, 1H); 13C NMR (CD3OD, 100 MHz) in ppm: 6 33.9, 65.2, 11.6, 121.4, 122.2, 127.7, 133.3, 155.8, 173.3;
References:
WO2012/6068,2012,A2 Location in patent:Page/Page column 99-100