
3-(2,6-Dibromophenyl)propanoic acid synthesis
- Product Name:3-(2,6-Dibromophenyl)propanoic acid
- CAS Number:957211-37-9
- Molecular formula:C9H8Br2O2
- Molecular Weight:307.97
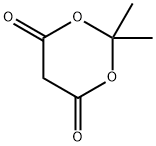
2033-24-1
631 suppliers
$6.00/25g
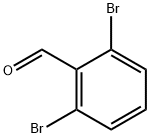
67713-23-9
181 suppliers
$15.00/1g

957211-37-9
14 suppliers
inquiry
Yield:957211-37-9 854 mg
Reaction Conditions:
Stage #1: cycl-isopropylidene malonate;2,6-dibromobenzaldehydewith formic acid;triethylamine for 4 h;Reflux;
Stage #2: with hydrogenchloride;water pH=Ca. 1;Cooling;
Steps:
3-(2,6-Dibromophenyl)propanoic acid (23-1)
To a solution of formic acid (2 mL) at 5 °C was added triethylamine (2.67 g, 26.4 mmol) drop- wise maintaining the temperature below 10 °C. Subsequently, 2,6- dibromobenzaldehyde (Intermediate 12-1) (1.0 g, 3.78 mmol) 2,2-dimethyl-l,3-dioxane-4,6- dione (652 mg, 4.53 mmol) were added to the solution and the mixture was refluxed for 4 h. Afterwards the mixture was cooled to an ambient temperature and poured onto ice-cold water (8 mL). The resulting suspension was acidified by 5.5M HC1 until pH l and stored in a refrigerator overnight. The precipitated crystals were filtered with suction, washed with water (3 x 4 mL) to provide 3-(2,6-dibromophenyl)propanoic acid (854 mg, 2.55 mmol) as a yellow solid. MS (ESI): m/z 309.1 [M + H]+, NMR (400 MHz, Methanol-^): δ 7.59 (d, J= 8.0 Hz, 2 H), 7.03 (t, J= 8.0 Hz, 1 H), 3.32-3.28 (m, 2 H), 2.55-2.51 (m, 2 H).
References:
WO2019/28165,2019,A1 Location in patent:Paragraph 0668; 0670-0671
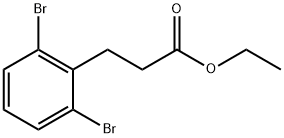
957211-36-8
0 suppliers
inquiry

957211-37-9
14 suppliers
inquiry
108-36-1
449 suppliers
$10.00/1g

957211-37-9
14 suppliers
inquiry