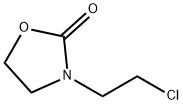
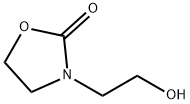
3-(2-chloroethyl)oxazolidin-2-one synthesis
- Product Name:3-(2-chloroethyl)oxazolidin-2-one
- CAS Number:2508-01-2
- Molecular formula:C5H8ClNO2
- Molecular Weight:149.58
Yield:2508-01-2 98.9%
Reaction Conditions:
with triethylamine in methanol at 20; for 1 h;
Steps:
1.1 Step 1: Synthesis of 3-(2-Chloroethyl)-2-oxazolidinone (Structural Formula (6-1))
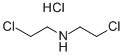
This step was carried out in air. To a 2 L four-necked round-bottom flask, a magnetic stirrer bar and a thermometer were attached, and N,N-bis(chloroethyl)amine hydrochloride (200.0 g, 1.12 mol, 1.0 equivalents), methanol (MeOH) (600 mL), and triethylamine (Et3N) (328.0 mL, 2.35 mol, 2.1 equivalents) were sequentially introduced. Carbon dioxide (CO2) gas generated from dry ice was passed through the obtained solution at room temperature for 1 hour. After the reaction liquid was concentrated under reduced pressure, toluene (1.0 L) was added, and the obtained white suspension was filtered by suction. Then, the residue was washed with toluene. The filtrates were collectively concentrated under reduced pressure to obtain 165.7 g of title compound (6-1) as a light yellow liquid. Isolated yield: 98.9%. Note that this compound could be decolorized by distillation purification (boiling point: 135° C. (3 mmHg)); however, this compound was used in the subsequent step without any further purification, because the compound was almost pure based on the results of NMR analyses.1H NMR (300 MHz, deuterated chloroform (CDCl3)): δ=4.38 (ddd, J=0.9, 6.3, 7.8 Hz, 2H), 3.79-3.67 (m, 4H), 3.66-3.59 (m, 2H). 13C NMR (75 MHz, CDCl3): δ=158.38, 62.01, 46.19, 45.70, 42.03.
References:
US2017/233418,2017,A1 Location in patent:Paragraph 0188; 0189; 0190; 0191; 0192; 0193
3356-88-5
50 suppliers
$60.00/500mg

2508-01-2
32 suppliers
inquiry

107-04-0
269 suppliers
$9.00/1g

2508-01-2
32 suppliers
inquiry
101973-67-5
3 suppliers
inquiry

2508-01-2
32 suppliers
inquiry